Special Report: How secrecy in U.S. courts hobbles regulators
 Send a link to a friend
Send a link to a friend
 [January 16, 2020]
By Mike Spector, Jaimi Dowdell and Benjamin Lesser [January 16, 2020]
By Mike Spector, Jaimi Dowdell and Benjamin Lesser
LEBANON, Ohio (Reuters) - Something wasn’t
right with the Rhino.
Reports started trickling in to the Consumer Product Safety Commission (CPSC)
in 2005 of people being killed or injured in the Yamaha Motor Co
off-road vehicles when they tipped over. But no clear pattern emerged,
and in the rough and tumble off-road world, accidents are common. The
agency took no action.
Then, in 2007, ten-year-old Ellie Sand was killed in a Rhino when it
flipped in a cornfield in Warren County, Ohio. Her father, house painter
John Sand, started reading up on other Rhino crashes. He spoke to other
parents whose children had died or been seriously injured in similar
incidents. He became convinced that Rhinos were the problem.
Using a computer in a Cincinnati law library, Sand found more than a
dozen lawsuits alleging that the vehicles were dangerously unstable.
Some of the lawsuits – like the one he filed against the company in 2008
– claimed that design flaws caused the Rhinos to roll over even at slow
speeds on flat ground. Sand sent the results of his research to CPSC,
highlighting details from the lawsuits of the tip-overs that led to
deaths and injuries.
Soon after, CPSC sent a subpoena to Yamaha, forcing it to hand over a
trove of information, much of which had lain hidden under judges’
protective orders in the lawsuits against the company. By then, more
than 40 people, including more than a dozen children, had been killed in
Rhino crashes.
The impact of Sand’s work was confirmed in a voicemail message Sand
received a few months later. Yamaha had agreed to a voluntary recall
covering more than 100,000 Rhinos to fix the stability problems. “As a
result of the information – a lot of the information that we received
from you – we’ve gotten this far, so I wanted to thank you,” Marc Schoem,
then deputy director of CPSC’s compliance and field operations, said in
the March 31, 2009, message, which was reviewed by Reuters.
Five years after the recall was announced, CPSC staff noted in a 2014
briefing that crashes involving the Rhino had “decreased noticeably.”
Sand told Reuters he “was elated that the work I’d done could help stop
the carnage.” Still, he was surprised that it took an “average Joe” like
him to flag the lawsuits to CPSC.
A CPSC spokesman said that the agency had investigated Rhino incidents
and that establishing a pattern that would have supported finding the
vehicles defective proved challenging. Schoem declined to comment, as
did Yamaha.

ERRORS AND INCONSISTENCIES
CPSC is one of more than a dozen regulatory agencies tasked with
protecting Americans from dangerous products. And the Rhino episode
reveals a troubling dynamic in the way these watchdogs do their jobs:
Sometimes the only way they can learn about and act on a possible threat
to consumers is from evidence produced in lawsuits, but that evidence is
often hidden behind a wall of secrecy.
Most regulators have their own reporting systems for conducting
oversight. But their databases can be vast and unwieldy, stuffed with
thousands and even millions of consumer complaints and reports from
manufacturers of safety concerns, injuries or deaths. The reports are
often rife with mistakes and inconsistencies. Not all consumers even
know they can file complaints. And companies regularly flout legally
mandated reporting requirements.
That leaves the courts as a conduit for alerting regulators to potential
harm, and it’s far from perfect. As Reuters has documented in earlier
articles in this series, a thick blanket of secrecy covers
product-liability litigation in the United States. In just a handful of
cases over the past several decades, hundreds of thousands of people
were killed or injured by defective products – cars, drugs, guns – while
information about the risks was hidden from consumers and regulators,
sometimes for years, behind broad protective orders.
These orders, though meant to protect specific information such as
medical records and trade secrets, often give companies wide latitude to
designate as confidential material exchanged between litigants in the
pretrial discovery process – internal emails, data, research, meeting
minutes, sworn depositions and the like. The secrecy typically persists
for the life of the case, and long after, though court documents are, by
law, presumed to be public.
In an analysis of some of the largest mass defective-product cases
consolidated in federal courts over the past 20 years, Reuters found 55
in which judges sealed information concerning public health and safety.
And among those, only three had protective orders containing language
specifically allowing information exchanged by the litigants to be
shared with regulators.
Regulators may subpoena information from a manufacturer after spotting a
suspicious cluster of lawsuits, or after being alerted by a consumer
like Sand in the Yamaha Rhino case.
But those are rare exceptions. And regulators themselves aren’t inclined
to mine court records as a means of oversight. In the 55 big cases
Reuters reviewed, public court filings contained no indication that
regulators had requested any information arising from the lawsuits.
A few years ago, the National Highway Traffic Safety Administration (NHTSA)
and CPSC issued pleas for easier access to evidence introduced in court
under protective orders. But the Environmental Protection Agency, the
Food and Drug Administration (FDA) and 15 other federal departments or
agencies surveyed by Reuters did not point to any explicit policy or
guidance on gaining access to court evidence potentially relevant to
their oversight functions.
Yet regulators have repeatedly documented the failures of existing
safeguards. Since 2009, NHTSA and CPSC have fined a total of at least 90
companies for failing to meet safety-reporting requirements, while the
FDA has issued more than a dozen warning letters to manufacturers of
drugs and medical devices for similar lapses.
Big business and its lobbyists contend that regulators have all the
tools they need to do their jobs well – including the power to subpoena
information subject to a judge’s protective order.
“The protective order cannot block the government,” said Victor
Schwartz, a partner at law firm Shook, Hardy & Bacon LLP who has
defended companies in civil litigation. “Litigation gets publicity. If
the government sees something about a case … it can use its power, the
subpoena power, to find out more detail,” he said.
That argument, former U.S. regulatory officials said, doesn’t hold water
when litigation is cloaked in secrecy. “It’s a catch-22,” said David
Friedman, a former NHTSA official. If documents and other evidence in
litigation are sealed, “how are you supposed to know about them?”
Friedman said. “If you don’t know about them, you can’t get them.”
DEFECTIVE SWITCH
Friedman was at NHTSA during General Motors Co’s notorious 2014 recall
of millions of cars with defective ignition switches, ultimately linked
to 124 deaths and 275 injuries. A 2015 deferred prosecution agreement
between GM and federal prosecutors showed the company scrambled for
years to make sense of mounting reports of deaths and injuries while
keeping regulators and the public in the dark about the switches, even
after uncovering clear internal evidence they were defective.
As early as 2003, NHTSA received complaints that Saturn Ions were
stalling and, by 2004, that their airbags were failing to deploy in
collisions. Similar complaints soon cropped up about Chevrolet Cobalts.
In the ensuing years, the agency examined several fatal Cobalt crashes,
each involving switches that slipped out of position and disabled
airbags.
Yet NHTSA didn’t make the connection between the switch problem and
airbag failures. That was partly because its investigators misunderstood
how GM’s airbag system operated, but also because NHTSA rules gave
automakers a lot of leeway in how they reported certain information
regarding safety risks. As a result, similar incidents were reported
inconsistently. One was listed as an “engine and engine cooling” issue,
for instance, while another as an “electrical” problem. That made it
difficult for regulators to detect a pattern.

From the first consumer complaints and incident reports, it would be
more than 10 years and scores of deaths and injuries before NHTSA and
the public learned of the link between defective ignition switches and
airbag failures – and then only after evidence of what GM knew emerged
in litigation and prompted the automaker to pursue a recall.
In June 2011, the parents of Jennifer Brooke Melton filed a
product-liability lawsuit against the company in a Georgia state court.
Melton, a 29-year-old nurse, was killed in March 2010 when her Cobalt
stalled on a rainy highway, crossed into oncoming traffic, collided with
another vehicle and careened into a creek.
The Melton case started, as many like it do, under a veil of secrecy. In
December 2011, Judge Kathryn Tanksley approved a broad protective order,
keeping from the public and NHTSA any documents that GM designated “in
good faith” as confidential.
Tanksley, now retired, said she approved the order because both the
Meltons’ lawyer and GM agreed to the terms. “The role of litigation is
not to regulate GM,” she said. When NHTSA officials want more
information, she said, they “have to pursue it not through the court,
but through their own power.”
The documents GM began turning over to Lance Cooper, the Meltons’
lawyer, were damning. They showed that in 2005, for example, a company
engineer suggested a fix for less than $1 per vehicle, but it was
rejected as too costly and not effective enough. Cooper also obtained
evidence that the company modified the switches between 2005 and 2008 to
keep them from slipping. The evidence built a strong case that GM had
known for years that the switches were faulty.
The Meltons were eager to go public with the evidence to prevent others
from dying as their daughter had, but Cooper worried that if he
challenged the protective order and regulators didn’t conclude the
switches were defective, it would hurt their case. GM wanted to settle.
“We thought that people needed to know. There were still people out
there driving those cars,” Beth Melton told Reuters. “It’s a real shame
that these things are kept secret and other people (suffer) because of
it.”
Meanwhile, a Cobalt crash in Quebec, Canada, killed another driver. The
airbags did not deploy. The ignition switch was later found to be in
accessory mode, the position between on and off that could cut power to
airbags.
GM settled with the Meltons in September 2013 for $5 million. Five
months later, after conducting its own investigation, GM recalled about
600,000 vehicles with the ignition switch.
Cooper still wasn’t satisfied, based on what he had learned in the
Melton lawsuit, and having obtained a settlement for his clients, he
decided it was time to share evidence with NHTSA. “I basically said:
‘The hell with it,’ ” Cooper said. “If we can get this information to
the federal government, they need it. Really, it was just a strategic
decision to violate the protective order.”
In a letter to NHTSA , he suggested that the automaker knew about the
defect far longer than its recall paperwork said and had not recalled
enough vehicles. He urged the agency to investigate, citing evidence
from the Melton case that he had seen as much as a year earlier.
[to top of second column]
|
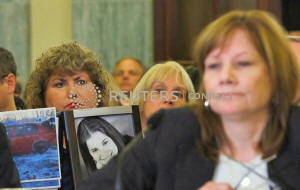
Mary Theresa Ruddy (L), whose daughter Kelly was killed in 2010 when
she lost control of her 2005 Chevrolet Cobalt, holds pictures of the
vehicle and Kelly, as General Motors CEO Mary Barra testifies before
the Senate Commerce and Transportation Consumer Protection, Product
Safety and Insurance subcommittee in Washington April 2, 2014. To
match Special Report USA-COURTS/SECRECY-REGULATORS REUTERS/Gary
Cameron/File Photo

Eventually, NHTSA, Congress and federal prosecutors all
investigated, relying heavily on evidence from the Melton case. GM
increased the size of the recall, which eventually covered 2.6
million vehicles.
In May 2014, NHTSA fined GM $35 million for failing to alert
regulators to the defective ignition switch in a timely manner. The
next year, GM entered into the deferred prosecution agreement with
the U.S. Attorney’s Office for the Southern District of New York to
settle criminal charges of concealing information from government
officials and wire fraud. Under the deal, GM agreed to pay a $900
million fine and to submit to three years of oversight by an
independent monitor. In 2018, a federal judge dismissed the charges
against the company after prosecutors said the company had complied
with the agreement.
“The most damning information came out in litigation,” said Kevin
Vincent, NHTSA’s head lawyer at the time. Though the facts
eventually came to light, Vincent said, initial confidentiality in
the Melton case “stymied” the agency. “That was evidence the agency
needed to see,” he said. “We could have acted sooner.”
In a statement to Reuters, GM said: “Since 2014, we have undertaken
comprehensive reforms across the company to ensure that something
like the ignition switch crisis never happens again.” The company
hired additional safety investigators, created a new executive
position charged with overseeing global safety and recalls, and
launched a program aimed at giving employees and dealers easier ways
to flag potential vehicle defects.
A NHTSA spokesman said the agency took 17 steps the U.S.
Transportation Department inspector general recommended in the wake
of the ignition-switch recall to improve collection and analysis of
vehicle safety data.
PLEAS FOR ACCESS
In 2016, NHTSA and CPSC, seeking to address what they acknowledge is
a blind spot in their efforts to safeguard consumers, issued
bulletins recommending that judges and litigants agree to protective
orders that would allow them to share confidential evidence
pertinent to public health and safety with the relevant regulators.
“Our job is to protect consumers,” said Marietta Robinson, a CPSC
commissioner at the time. “Obtaining information about an allegedly
dangerous product from a lawyer representing a consumer who has been
injured or killed is critically important to us doing that job.”
NHTSA’s bulletin, which cited the GM ignition-switch case, argued
that keeping such information hidden from regulators clashes with
federal legal requirements for courts to show “good cause” before
allowing companies to keep it secret.
But the bulletins, published in the Federal Register, where the
government publishes new rules, proposals and public notices,
carried no enforcement power, and they have had little impact.
Judges have rarely shown willingness to grant requests from
plaintiffs, expert witnesses or news organizations to share
information with regulators or the public. Lawyers challenged
defendants’ claims of confidentiality for material relating to
public health and safety in 26 of the 55 big cases Reuters analyzed,
and in most of them, judges refused to unseal the evidence. Some of
those cases involved attempts to share information with the FDA.
The FDA declined to comment on specific cases for this article. In
general, the agency said, its powers to inspect drug makers' plants
and launch criminal investigations, along with voluntary reporting
requirements for drug makers, “provide FDA with the tools to keep
patients and consumers safe and fulfill its mission to protect and
promote the public health.”
However, in the case of Pfizer Inc’s popular drug Chantix, which
helps people quit smoking, court secrecy excluded possibly pertinent
information from the agency’s process for assessing safety.
The FDA approved Chantix in 2006. Three years later, it placed a
black box warning – its strongest – on the drug’s label after
receiving “reports of changes in behavior such as hostility,
agitation, depressed mood, and suicidal thoughts or actions.”
Over time, Pfizer faced thousands of lawsuits blaming Chantix for
such side effects. The company turned over millions of documents to
plaintiffs under the condition they be kept confidential.
In 2014, after Pfizer settled most of the cases for about $300
million, two plaintiff experts decided the FDA and the public should
see internal Pfizer documents and expert reports that had been
introduced in litigation.
Through their lawyer, clinical psychiatrist Joseph Glenmullen and
drug safety researcher Thomas Moore asked the judge overseeing the
bulk of the Chantix litigation to unseal the information, which they
said was important for “shedding light on Pfizer’s awareness of
Chantix’s behavioral risks.”
Two days after their request was filed, U.S. District Judge Inge
Johnson in Alabama rejected it. Her brief order did not address the
substance of the request.

Johnson did not respond to requests for comment.
The upshot was that, two years later, an FDA advisory panel did not
have access to all the information the two men had sought to make
public as it considered a Pfizer request to remove the black box
warning. Pfizer’s request was based on its own study claiming that
Chantix did not have a significant association with depression and
suicide.
The advisory panel of medical experts in September 2016 recommended
in a close vote to remove the black box warning. The FDA removed the
black box warning a few months later. Chantix bottles still carry a
less severe warning of potential mental health side effects.
Moore acknowledged that it’s impossible to know whether the evidence
he and Glenmullen sought to provide to regulators would have changed
the panel’s decision. But he said he remains frustrated that
information “central” to the question of Chantix’s safety never made
it into regulators’ hands.
“The FDA should be able to see it,” Moore said.
Pfizer, in a statement to Reuters, said: “The FDA and its 2016
advisory panel had access to all of the data and science on Chantix,
and all of the adverse events reports.” The plaintiff experts’
reports, the company noted, were not original science and instead
reflected views of the underlying science that differed from the FDA
and the advisory panel’s conclusions.
Members of the FDA advisory panel Reuters contacted said they were
unaware of Moore and Glenmullen’s efforts.
One of them was Dr Jess Fiedorowicz, director of the University of
Iowa Mood Disorders Center. He voted to remove the black box
warning. He said he didn’t know whether the evidence from the two
experts would have changed his mind, but, “I’m all for transparency
in research.”
SEEKING SUNSHINE
In September, House Judiciary Committee Chairman Jerrold Nadler, a
New York Democrat, said he planned to reintroduce the Sunshine in
Litigation Act to address the problem of court secrecy. The bill
would allow parties in litigation to share evidence related to
public health and safety with state and federal regulators,
regardless of protective orders.
Nadler’s pledge came during a hearing on courtroom transparency that
was called after Reuters began publishing its series on court
secrecy and its impact on public health and safety. Previous
iterations of the bill introduced repeatedly since the early 1990s,
despite enjoying bipartisan support, have ultimately failed in the
face of sustained opposition from business groups that contend it
would increase the costs and burdens of litigation for companies
that are already meeting regulatory reporting requirements.
However, as the Yamaha Rhino episode and others like it show,
regulators and the public can’t assume manufacturers are meeting
disclosure and reporting rules.
A few months after John Sand sent a packet stuffed with his research
to CPSC, the agency told Yamaha it had received information
indicating that the Rhino could roll over at low speeds on flat
ground, posing “an unreasonable risk of injury or death to riders.”
It told the company to send it all relevant information, including
documents from Rhino-related litigation.
CPSC spent the next few months trying to get Yamaha to comply, at
one point complaining to the company that it was sending
“duplicative” material and that “the only new item seems to be a
Model Year 2009 owner’s manual for the Rhino.”
Only after CPSC subpoenaed Yamaha did the company send to the agency
a 70-page written response, a hard drive and 62 DVDs that contained
all of the records the company had produced in years of litigation,
including company documents that had been subject to protective
orders.
CPSC would have known about the Rhino much earlier if Yamaha hadn’t
repeatedly violated the rules for notifying the agency of a possible
defect, according to William Kitzes, a former adviser to the agency
and an expert witness for plaintiffs in Rhino litigation who
reviewed correspondence between the regulator and Yamaha.
Companies must report to the agency immediately upon learning that a
product is defective or can cause injury or death, or when a product
is subject to three or more personal-injury lawsuits in a two-year
period that are settled or decided in favor of plaintiffs. From 2004
to late 2008, Yamaha faced about 250 lawsuits alleging that Rhinos
were unsafe.

“Certainly no later than, and by many measures well before the end
of 2005 ... Yamaha had adequate information to report,” Kitzes wrote
in a report for plaintiffs.
CPSC didn’t fine Yamaha for failure to report Rhino incidents. The
agency and Yamaha jointly announced the Rhino recall on March 31,
2009 – referred to at the time, on Yamaha’s insistence, as a “free
repair program.”
In 2011, the Ohio jury hearing Sand’s case found the Rhino
defective, but awarded Sand no damages. Ellie was not wearing a
helmet when she was killed, and the jury determined that she, her
parents and others were negligent in her death.
Yamaha stopped making the vehicles in 2013.
(Additional reporting by Erica Evans and Dan Levine. Edited by Janet
Roberts and John Blanton.)
[© 2020 Thomson Reuters. All rights
reserved.] Copyright 2020 Reuters. All rights reserved. This material may not be published,
broadcast, rewritten or redistributed.
Thompson Reuters is solely responsible for this content. |