U.S. employers wary of coronavirus 'immunity' tests as
they move to reopen
 Send a link to a friend
Send a link to a friend
 [May 15, 2020] By
Caroline Humer and Timothy Aeppel [May 15, 2020] By
Caroline Humer and Timothy Aeppel
NEW YORK (Reuters) - U.S. employers have
cooled to the idea of testing workers for possible immunity to the
coronavirus as they prepare to reopen factories and other workplaces.
Blood tests that check for antibodies to the new coronavirus have been
touted by governments and some disease experts as a way to identify
people who are less likely to fall ill or infect others. Italian
automaker Ferrari NV has made antibody testing central to its “Back on
Track” project to restarting factories.
But many U.S. companies are not planning to use them, relying on face
masks, temperature checks, social distancing, and diagnostic tests for
those with symptoms, employers and healthcare experts told Reuters.
Mercer, which advises companies on healthcare benefits, has surveyed
more than 700 U.S. employers in industries from high tech to retail to
energy, and found 8% of companies said they would include antibody tests
in plans to screen employees.

Interest in antibody tests from employers has fallen in recent weeks as
reports have suggested that it is too early to conclude that antibodies
to the new coronavirus translate into immunity. The American Medical
Association cautioned on Thursday that these tests do not determine an
individual's immunity.
"Many employers ... are realizing that antibody testing isn’t going to
be a silver bullet and really isn't going to bring them any value," said
David Zeig, a lead consultant on clinical services at Mercer.
Other employers worry about their liability if they administer and
interpret such tests, or are concerned about test costs and
availability. Some were spooked by a flood of tests that hit the market
before being reviewed by regulators for accuracy, which has contributed
to confusion over results.
A new antibody test from Roche Holding AG that has shown itself to be
highly accurate could potentially help answer questions about antibodies
and immunity and change corporate demand, but it has not done so yet,
consultants and companies said.
Governments, however, are interested in antibody tests, particularly if
they are accurate. Britain on Thursday said it is in talks with Roche
over buying tests that it could use to create a certificate of immunity
once there is a better understanding of the science.
Collective Health, a healthcare technology company that has built
back-to-work strategies for large companies, is advising employers to
use diagnostic tests, not antibody tests.
"There has been a proliferation of low-quality antibody tests and the
antibody tests themselves don't necessarily answer any questions about
immunity," said Rajaie Batniji, Collective Health’s chief health
officer.
GETTING BACK TO WORK
When General Motors Co, Ford Motor Co and Fiat Chrysler Automobiles NV
reopen production next week, they intend to offer diagnostic tests to
workers, not antibody tests. Officials at the Detroit carmakers said it
was because it was not clear what the antibody tests show.
[to top of second column] |
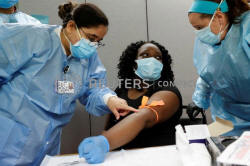
Shalonda Williams-Hampton, 32, has her blood taken by Northwell
Health medical workers for the antibody tests that detect whether a
person has developed immunity to the coronavirus disease (COVID-19)
at the First Baptist Cathedral of Westbury in Westbury, New York,
U.S., May 13, 2020. REUTERS/Shannon Stapleton

Amazon.com Inc’s on-site testing plan, now in development, does not include
antibody testing. Those views were echoed in interviews with a handful of
smaller U.S. manufacturers.
Shawn Kitchell, chief executive of Florida-based plastics manufacturer Madico
Inc, is not planning to use antibody tests for his 250 employees. He questions
their costs, accuracy, and the fact that the timing of tests can lead to
different results, requiring multiple tries.
"How frequently would we need to test to make it safer for our co-workers?"
Kitchell said.
Employers are also wary of an unregulated U.S. market for antibody tests. Since
March, the U.S. Food and Drug Administration (FDA) has allowed more than 200
tests into the market without regulatory review to make them available quickly,
opening the door to questionable vendors and inaccurate tests, Reuters found.
Last week, the agency set a deadline for all vendors to prove to the FDA that
their tests work or remove them from the market. It has also authorized two
highly-accurate tests from Roche and Abbott Laboratories, which are able to
supply millions of tests per week.
One of the biggest U.S. testing providers, LabCorp, on Thursday said it was
rolling out a program to make diagnostic tests and antibody tests available at
workplaces.
LabCorp’s chief medical officer, Brian Caveney, said interest in antibody
testing is coming from companies in coronavirus hotspots, such as New York,
while other areas with fewer COVID-19 cases see diagnostic testing as more
important.
As the new FDA process shows which tests work and which don't, that will help
advance research on how many people recovering from COVID-19 develop antibodies
and at what level, and show if they are truly immune to infection, said Howard
Koh, a professor at the Harvard T.H. Chan School of Public Health.

“Until we go through those steps, I don’t see how we can translate this for the
typical person who wants to go back to work,” Koh said.
(Reporting by Caroline Humer, Timothy Aeppel and Krystal Hu in New York and Ben
Klayman in Detroit; editing by Michele Gershberg and Nick Zieminski)
[© 2020 Thomson Reuters. All rights
reserved.] Copyright 2020 Reuters. All rights reserved. This material may not be published,
broadcast, rewritten or redistributed.
Thompson Reuters is solely responsible for this content. |