|
 UK
halts use of Silimed breast implants amid contamination
fears UK
halts use of Silimed breast implants amid contamination
fears
 Send a link to a friend
Send a link to a friend
[September 24, 2015]
By Kate Kelland
LONDON (Reuters) - Britain's health
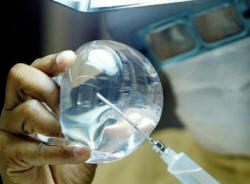
regulator has suspended sales of silicone implants made by Brazil's
Silimed due to contamination, and recommends none of the devices -
including breast, penile and testicular implants - be used until further
notice.
|
|
 The Medicines and Healthcare Products Regulatory Agency (MHRA) said
it and other European regulators are testing Silimed's products
after contamination was detected during an audit of the company's
manufacturing practices. The Medicines and Healthcare Products Regulatory Agency (MHRA) said
it and other European regulators are testing Silimed's products
after contamination was detected during an audit of the company's
manufacturing practices.
A German authority appointed to monitor Silimed "has recently
carried out an inspection of the manufacturing plant in Brazil and
established that the surfaces of some devices were contaminated with
particles," it said in a statement.
The Silimed suspension covers devices used in plastic surgery such
as breast and pectoral implants, urological devices including
testicular and penile implants and vaginal stents, as well as other
surgical devices, the MHRA said.
A spokesman for the MHRA said it did not yet know whether any
potentially contaminated products would have reached patients, and
did not know how many Silimed products might be affected. "All those
questions are part of our current investigations," he said.

The MHRA statement said however that "for the moment there has been
no indication that these issues would pose a threat to the implanted
person's safety."
"EU health regulators have initiated testing of samples of products
to establish if there are any health risks," it said.
Silimed, which says on its website it is the third largest implant
maker in the world, said in an email to Reuters it was preparing a
technical note to show its products are in compliance with national
and international norms and standards, and will send it to European
health authorities.
The Silimed product suspension comes after medical authorities found
in 2010 that one of the world's leading breast implant makers,
France's Poly Implant Prothèse (PIP), was not using medical-grade
silicone in its devices, leading them to have double the rupture
rate of other implants.
[to top of second column] |

Hundreds of thousands of patients across Europe and South America
were affected, and PIP's president, Jean-Claude Mas, was given a
four-year jail sentence in December 2013.
The British Association of Plastic, Reconstructive and Aesthetic
Surgeons (BAPRAS) said it was aware of the Silimed issue and was
working closely with British regulators.
"There has been no indication...that these issues pose a threat to
patient safety, however we are advising our members to contact any
patients who may be affected," BAPRAS president Nigel Mercer said in
a statement.
He said any patients with concerns about implants should seek advice
from their surgeon or clinic.
(Additional reporting by Anjali Rao Koppala, Abhirup Roy and Brad
Haynes; Editing by Adrian Croft)
[© 2015 Thomson Reuters. All rights
reserved.] Copyright 2015 Reuters. All rights reserved. This material may not be published,
broadcast, rewritten or redistributed.
 |