Blue-blooded crabs at heart of pharma dispute on drug testing
 Send a link to a friend
Send a link to a friend
 [August 01, 2019]
By John Miller [August 01, 2019]
By John Miller
ZURICH (Reuters) - If you've received a
vaccination, hip replacement or chemotherapy without suffering toxic
shock, chances are you've benefited from a vast bloodletting of
horseshoe crabs.
Every year, fishermen net hundreds of thousands of the creatures off the
U.S. East Coast and in Asia before their prized milky-blue blood is
drained for use in medical safety tests.
Swiss biotech Lonza <LONN.S> and U.S.-based Charles River Laboratories <CRL.N>
are the biggest suppliers of crab blood-based endotoxin tests, which
detect bacterial contamination in intravenous drugs and medical
implants.
They are now at odds over the future of this testing, as Lonza urges
adoption of a synthetic alternative called recombinant Factor C (rFC),
amid pressure from wildlife campaigners and worries about supply
reliability.
Meanwhile Charles River, which is still studying rFC, argues moving too
quickly could compromise patient safety.
There's much at stake - for both companies, the industry and, of course,
horseshoe crabs, a 450-million-year-old species that's more closely
related to scorpions than crustaceans.

Endotoxin tests number 70 million annually and are rising. If rFC gains
traction, it could re-shuffle winners and losers in the global market
for such testing that is seen growing about 13% a year to close to $700
million in annual revenue by 2021 and $1 billion by 2024, according to
market researchers.
For Lonza, whose internal testing growth estimates are more modest, the
risk is that the synthetic method won't catch on, hurting efforts to
capitalize on a $460 million investment it made in 2006 when it bought
the technology along with a peer's biological businesses.
Conversely, Charles River risks becoming a laggard if the technique wins
wide acceptance.
PHARMACOPOEIA PUSH
The leading U.S. pharmaceuticals standards organization, U.S.
Pharmacopoeia (USP), which publishes a compendium of drug information,
told Reuters it was pressing ahead to recognize synthetic tests as
comparable to crab blood-based ones.
"There is a consensus on moving forward and USP is fully committed,"
said Fouad Atouf, USP's Science-Global Biologics head.
"Making sure all the stakeholders are on board and all the data has been
collected is what's happening right now," he said, with proposed
revisions recognizing rFC due around September for public consultation.
That coincides with a push by USP's European counterpart in Strasbourg,
to recognize rFC. Japan is also working on the issue.
France's Biomerieux <BIOX.PA> and Germany's privately held Haemochrom
Diagnostica, which have versions of rFC, are also eager to see
pharmacopoeias act.
Lonza said its rFC product, PyroGene, now accounts for less than 5
percent of its endotoxin test sales but sees fortunes changing as global
pharmacopoeias act.
"We would hope to see around 10% by 2021," Lonza said.
It has won a big customer for PyroGene: Eli Lilly's <LLY.N> migraine
medicine Emgality last year became the first rFC-tested drug to win U.S.
regulatory approval.
Roche <ROG.S> and Bayer <BAYGn.DE> told Reuters they were also
considering rFC.
'UNACCEPTABLE RISK'
Charles River insists additional scrutiny is necessary before rFC
technology wins equal standing.
Companies pushing rFC must prove it can detect endotoxins in the real
world, not just the manufactured strains used so far in studying the
synthetic agent, said John Dubczak, Charles River's microbial solutions
manager.

"A move by the pharmaceutical industry towards current rFC methods is
premature," said Dubczak, adding more data is needed.
Lonza points to analyses by multiple drugmakers demonstrating rFC was
comparable to the crab-blood tests in ensuring drugs were
contaminant-free.
To be sure, Lonza has no plans to stop bleeding crabs, since many
existing medical products will continue to be tested with traditional
methods, it said.
The industry predicts the global crab blood-based test market will also
expand in the next few years.
But Lonza is counting on rFC emerging as a new pillar of its testing
business, contributing to its goal of more than 7 billion Swiss francs
($7.1 billion) in group annual revenue by 2022, from 5.5 billion francs.
[to top of second column]
|
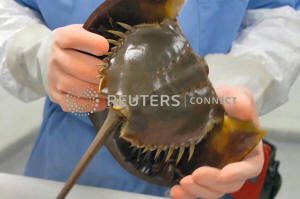
A technician holds a horseshoe crab at a Lonza biotech facility in
Walkersville, Maryland in an undated still image from video. Lonza/Handout
via REUTERS.

NATURE'S INGENUITY
Horseshoe crabs' copper-rich blood has helped them survive since
before the dinosaurs in brackish coastal waters by clotting around
endotoxins, the potentially dangerous molecules found in cell walls
of bacteria like E. coli.
Scientists harnessed nature's ingenuity, using crab blood to make
so-called amebocyte lysate endotoxin tests which, by the 1970s,
began displacing tests on rabbits that were injected with medicine
then monitored for fever.
However, concerns have since escalated over wildlife conservation
and the supply chain's reliability.
In Asia, where horseshoe crabs are also eaten by humans, the
tri-spine species is listed by the International Union for
Conservation of Nature as at risk of extinction. Two other species
are also imperiled.
Glenn Gauvry, director of the Delaware-based Ecological Research and
Development Group which focuses on horseshoe crab conservation, said
Asian crabs destined for dinner tables were first "rented" to
biomedical companies for bleeding.
Gauvry contends the biomedical industry is perpetuating consumption
of crabs in Asia and contributing to their decline.
Lonza does not harvest Asian crab blood.
Charles River, which does, said it followed local rules.
"Horseshoe crab blood is collected only to a point where the crab
naturally stops bleeding," Dubczak said, adding its Asian suppliers
and local governments were responsible for ensuring crabs were
released into "waters of appropriate salinity".
RUFA RED KNOT
Studies paint a mixed picture of the blood harvest's effects on U.S.
East Coast horseshoe crabs, hundreds of thousands of which are also
killed annually for bait or caught incidentally by commercial
fishermen hunting other marine species.
Those caught by Lonza, Charles River and Associates of Cape Cod,
owned by Japan's Seikagaku Corp <4548.T>, are drained of about a
third of their blood, then returned alive to the sea.

An Atlantic States Marine Fisheries Commission report from May
indicates current harvest levels "appear to be sustainable".
Additionally, a recent U.S. Geological Survey study found bled
crabs' survival rates may exceed those of non-bled crabs in New
Jersey's Delaware Bay.
However, previous studies found anywhere between 4% to 30% of bled
crabs may die prematurely, prompting pressure on the biomedical
industry, largely out of concern for shorebird populations that rely
on crab eggs.
Advocates including the Audubon Society became increasingly vocal
when the rufa red knot, which eats crab eggs on stopovers at
Delaware Bay, was listed as threatened under the U.S. Endangered
Species Act in 2014.
"That was the tipping point," said Jay Bolden, an Eli Lilly
biologist whose hobby as a birdwatcher spurred him to champion his
company's switch to rFC that he believes will ensure supply-chain
reliability.
"If it got really serious, and they said nobody could touch the
horseshoe crabs because they have to rebuild the red knot
population, that was going to be significant."
Lilly has shifted three of eight global drug manufacturing sites to
rFC testing and is more than halfway to its goal of 90 percent rFC
testing by 2020, he added.
"Until the global pharmacopoeias revise the test method to recognize
rFC as on par with crab blood-based tests, we can't just make the
change for medicines that are already on the market," Bolden said.
"When that happens, then we can change over that final 10 percent."
(Reporting by John Miller; Editing by Michele Gershberg and Pravin
Char)
[© 2019 Thomson Reuters. All rights
reserved.]
Copyright 2019 Reuters. All rights reserved. This material may not be published,
broadcast, rewritten or redistributed.
Thompson Reuters is solely responsible for this content.
 |