Russian state employees describe pressure to join vaccine trials
 Send a link to a friend
Send a link to a friend
 [December 04, 2020]
By Polina Ivanova, Rinat Sagdiev, Gleb Stolyarov and Kate Kelland [December 04, 2020]
By Polina Ivanova, Rinat Sagdiev, Gleb Stolyarov and Kate Kelland
MOSCOW (Reuters) - In late September,
Moscow municipal official Sergei Martyanov sent a series of text
messages to his subordinates: "Colleagues!!!... What is this
sabotage???"
Martyanov was expressing dismay at his staff's apparent reluctance to
volunteer for the human trials of Russia's Sputnik V coronavirus
vaccine, named after the Soviet-era satellite that triggered the space
race. The official in the Moscow department of city property said many
quota spots for his staff to join the trial remained unfilled.
He said he had heard some workers were signing up to receive flu
vaccines, making them ineligible for the coronavirus trial.
"Who are you trying to trick???" Martyanov said in the texts. "The
coronavirus vaccine is the absolute priority!!!"
Anyone who had received the flu jab, he said, must still sign up for the
COVID trial, allowing a month's delay. He urged his colleagues to
recruit friends and family into the trials. "At least two people per
employee!"

Martyanov, the head of his department and Moscow's city administration
did not respond to requests for comment. The Moscow health department
said the vaccine has already successfully passed two stages of clinical
trials and has shown its safety, and the decision to participate in the
trial is made by residents only voluntarily and only after a medical
examination.
But the messages, seen by Reuters, reveal how some Russian state
employees are coming under heavy pressure to sign up for the trials, an
effort that medical ethicists say may run afoul of ethical norms for
voluntary participation in such tests.
A source close to Martyanov's department told Reuters that all
departments in Moscow's city administration, which employs around 20,000
people, were set quotas for participation in the trials.
Russia's vaccine testing began in early September and is in its final
phase in 29 clinics across Moscow. About 20,000 people have already
taken part. The government says interim results show the vaccine is 92%
effective. The country aims to produce more than a billion doses of the
shots at home and abroad next year.
Even before the trials have been completed, Russians are already
receiving the vaccine. The medicine received formal regulatory approval
from Russian authorities in August; Russia, which has the world's
fourth-highest number of recorded COVID-19 cases, says it has so far
inoculated more than 100,000 people considered at high risk such as
military personnel, doctors and teachers.
President Vladimir Putin has said the vaccine "passed all checks." Putin
himself has yet to be vaccinated, however: His position means he cannot
take something that is still being tested, the Kremlin says. In August,
Putin said one of his daughters had been inoculated and was fine
afterwards.
In conducting the trials, Moscow is helped by legions of Russian public
sector workers who rely on the government for their pay. Over three days
in November and six days in October, Reuters reporters visited 13 of the
trial clinics and spoke to 32 trial participants. Thirty of the 32 said
they had been told about the trial at work.
Of the 32, 23 people said they were genuine volunteers. Most expressed
enthusiasm for participating in the trial.
Nine said they were not true volunteers. All nine were public sector
workers who spoke on condition of anonymity. A few said they felt they
could not refuse their employers' entreaties to seek the vaccinations,
but that after they arrived, medical tests had shown them to be
ineligible, or staff gave them reasons they could use to opt out.

Some said they got as far as the clinic and then simply refused to take
part. None said they had been injected against their will.
Medical ethicists said the pressure being put on state employees may
nonetheless be stretching the norms of ethical testing guidelines.
Generally speaking, if people feel there's a cost to them if they refuse
to take part in a trial, that is coercion, which wouldn't be justified
in the United States, the UK or other Western countries, Oxford
University ethics professor Julian Savulescu told Reuters.
Jonathan Ives, a reader in empirical bioethics at Bristol University's
Centre for Ethics in Medicine in Britain, said that what counts as
coercive can depend on the relationship between those involved.
"Even if very light pressure is being put on an employee by an employer
to take part in a trial, and that employee feels their job or wellbeing
may be at risk if they do not accede to that pressure, I think this
would be coercive, and I would be very concerned about that," he said.
The Russian Direct Investment Fund, which is backing the vaccine's
development and responsible for its marketing abroad, declined to
comment.
Alexei Kuznetsov, an aide at the health ministry, which oversees the
Gamaleya Institute where the vaccine is being developed, said the
participation of volunteers in clinical trials "is possible only on a
voluntary basis."
"COMPULSORY"
With the vaccine already approved, some officials have outright ordered
staff to receive the shots.
"Our clinic has been issued with a compulsory anti COVID-19 vaccination
order for all employees. This is being supervised by the Moscow health
department," Olga Tsvetkova, deputy chief medical officer at Moscow's
Clinic Number 3, announced in a message to staff in October.
"If you refuse to get vaccinated, you could be suspended from work.
There is a legislative basis for this," she wrote in the WhatsApp
message seen by Reuters, without elaborating.
Asked to comment, Clinic Number 3 said Moscow is one of the few regions
where healthcare workers are given the opportunity to be vaccinated
against the coronavirus, and the order to vaccinate employees of the
clinic shows the degree of care it takes.
"The decision on vaccination is made by employees voluntarily and only
after passing a medical examination," it said in a statement.
Moscow's health department did not comment on that order.
Savulescu, the Oxford professor, said a vaccine can be ethically rolled
out while clinical trials are still underway if there is enough evidence
it is safe. "If you have reached that point, then, it is possible to
justify a mandatory policy," he said.
He added that without knowing the safety data on Sputnik V, it was not
possible to comment on Russia's decision. The trial organisers have said
there were no unexpected adverse events so far and monitoring of the
participants is ongoing, but detailed safety data has not been
published.
[to top of second column]
|
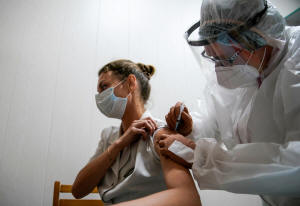
A medic of the regional hospital receives Russia's "Sputnik-V"
vaccine shot against the coronavirus disease (COVID-19) in Tver,
Russia October 12, 2020. REUTERS/Tatyana Makeyeva

Mandatory vaccination is common in the U.S. healthcare industry; the
U.S. Occupational Safety and Health Administration (OSHA) has said
in the past that employers have the right to mandate vaccines. In
Europe, there is a patchwork of national vaccine regulation. Some
countries mandate childhood vaccinations, but experts say employers
overall are unlikely to be able to do so for staff.
BUDZHETNIKI
The world community has established norms for ensuring ethical
participation in clinical trials. According to guidelines in the
World Medical Association Declaration of Helsinki, used by most
countries globally, individuals taking part in clinical trials must
be capable of giving consent, informed of all aspects of the trial
that are relevant to them, and taking part voluntarily.
Russia has adopted a different set of internationally agreed
guidelines - from the International Council for Harmonisation of
Technical Requirements for Pharmaceuticals for Human Use (ICH).
These also say participation must be voluntary.
The process includes signing an informed consent form. In Moscow,
participants sign a similar 16-page document which says
participation is voluntary, unpaid, and that "it is impossible to
exclude the possibility of the development of an unexpected
undesired effect."
Staff in Martyanov's department, like others who work for
institutions that are financed from the Russian state budget, are
known as 'budzhetniki,' or 'budgetniks.' Their ranks are often
described in Russia as a reliable tool for the Kremlin when it needs
large numbers of people to participate in projects such as voting in
elections or referendums.
Sociologist Denis Volkov, deputy director of the Levada-Center, an
independent opinion and sociological research organization, said
many Russians with government-funded jobs feel duty-bound to deliver
what the government wants as part of a social contract between them
and the state.

"You are asked by the state, and in exchange it takes care of you
and provides you with financial prosperity," he said. "It is a
delicate game. They won't force you but will persuade you, convince
and give recommendations."
According to recent estimates from the Federal State Statistics
Service, there are almost 19 million budzhetniki working in jobs in
areas such as schools, hospitals, or municipal hygiene in Russia.
That's 26% of Russia's working-age population of just under 72
million.
"HERDED IN"
At a clinic in Solntsevo, a neighbourhood of high-rise apartment
blocks on Moscow's outer edge, five people turned up to join the
trial over the course of an hour. All five, approached by Reuters,
said they didn't actually want to take part but had felt they had to
- they were among the nine public sector workers who said their
bosses had pressured them to take part.
"They herded us in here," one middle-aged emergency services worker
said. His whole team was told they had to sign up, he said. "It's
impossible to say no, you just can't."
Moscow emergency services said participation in vaccination trials
was "absolutely voluntary, there was no coercion against the
personnel," and so far 101 volunteers have been vaccinated.
A teacher said that the school where he worked had been allocated a
quota of trial spots, but it was more than simply an invitation to
take part.
"If they say you have to come, you have to come," the teacher said,
adding that 17 staff members signed up.
Two hospital workers said they had been sent by their employer. And
a worker with a street-cleaning company said he and his colleagues
had been told participation was compulsory because they come
face-to-face with city residents in the street.
Asked if he could decline, he laughed: "No, we work for the public
sector."
All five people at the clinic were ultimately deemed ineligible, so
they wound up not receiving the shots.

But one woman aged around 50 at Polyclinic Number 68 in central
Moscow took a different view. She said her employer had compelled
her to attend, but she had only turned up to exercise her right to
formally refuse a jab.
"I don't want to be a guinea pig," she said.
"NO DOWNSIDES"
Most Muscovites Reuters spoke to were keen to join the trials. Asked
about side effects, some volunteers who had already had one of the
two-jab doses variously described feeling drowsy and said their
temperatures briefly rose at the start. None reported any serious
impact.
At Clinic Number 68, a worker with state bank PJSC Sberbank, the
largest lender in Russia, said he was offered the vaccine at work
and that the first stage of the process - the medical exam - could
be done in the company's office. Sberbank said it had actively
supported the vaccine development and employees could volunteer for
trials but no medical exams took place in its offices.
"You don't need to go around the different clinics. If you want (to
be vaccinated) - go ahead. If you don't want to - so be it," he
said. "I didn't see any downsides."
Anton Shirkin, a park worker, said he decided to participate because
he frequently visits his elderly parents and because "ultimately,
someone has to do it."
(Reporting by Polina Ivanova, Rinat Sagdiev, Gleb Stolyarov in
Moscow and Kate Kelland in London; with additional reporting by
Maria Tsvetkova, Vladimir Soldatkin, Polina Nikolskaya and Anton
Zverev in Moscow and Marisa Taylor in Washington; Edited by Sara
Ledwith)
[© 2020 Thomson Reuters. All rights
reserved.] Copyright 2020 Reuters. All rights reserved. This material may not be published,
broadcast, rewritten or redistributed.
Thompson Reuters is solely responsible for this content. |