EU raises its bet on blood plasma in search for COVID-19 therapy
 Send a link to a friend
Send a link to a friend
 [July 09, 2020]
By Francesco Guarascio [July 09, 2020]
By Francesco Guarascio
BRUSSELS (Reuters) - The European Union
wants to fast-track funding to treat COVID-19 patients with blood plasma
collected from survivors, an EU document seen by Reuters shows, in a
sign of the bloc's growing confidence in the experimental treatment.
The move also highlights the more assertive approach being taken by the
27-nation union in the race to find effective drugs and vaccines against
the new coronavirus, after the United States scooped up several
promising candidates.
The European Commission, the EU's executive arm, has invited national
blood authorities to apply for possible emergency funding by July 10 to
boost their collection of convalescent plasma, which is obtained from
people who have recovered from COVID-19, the document seen by Reuters
said.

Funds could be used to buy equipment to collect, store and test
convalescent plasma, the document said, adding the money could come from
the Emergency Support Instrument (ESI), a European rainy-day fund.
The use of the ESI could allow funds to be provided this year. Usually
EU funding projects are planned years in advance.
Money from the 2.7-billion-euro ($3 billion) ESI has so far only been
used or committed for highly sensitive issues, such as buying scarce
face masks at the peak of the pandemic in Europe and advance purchase of
potential COVID-19 vaccines.
Over 300 million euros have been spent and about 2 billion is pencilled
in to buy possible vaccines, EU officials told Reuters. This leaves some
400 million euros available.
The use of the ESI is still being considered, the Commission noted in
its document. A Commission spokesman did not immediately reply to
questions on the matter.
PLASMA RUSH
Since the beginning of the pandemic, medics across the world have been
transfusing convalescent plasma into critically ill COVID-19 patients,
often with positive results, although its efficacy is still under
investigation.
[to top of second column]
|
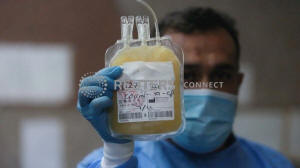
A nurse wearing a protective face mask and gloves shows blood plasma
from a person who has recovered from the coronavirus disease
(COVID-19), to be used to help critically ill patients, at a blood
bank in Basra, Iraq June 20, 2020. REUTERS/Essam Al-Sudani

People who survive an infectious disease like COVID-19 are left with
blood plasma containing antibodies, or proteins made by the body's
immune system to fight off a virus, that can be transfused into
newly infected patients to try to aid recovery.
Plasma, which is the liquid component of blood, is also being tested
by public authorities and companies to develop medicines against
COVID-19, such as hyperimmune globulins.
Separate research is underway on its possible use to prevent
COVID-19 infections, as antibodies extracted from it could be
transfused to boost immunity defences of vulnerable people. That
could be particularly important in the absence of a vaccine.
The Commission has already funded research on convalescent plasma,
but unblocking emergency funding to promote collection would be the
boldest move so far.
The EU is currently financing a project to develop a plasma-derived
therapy against COVID-19 and has also set up a database to share
results of treatments applied in European hospitals.
It is also working to reduce its long-standing dependency on plasma
imported from the United States to manufacture critical non-COVID
medicines such as immunoglobulins and medication that helps control
bleeding.

(Reporting by Francesco Guarascio @fraguarascio; Editing by Mark
Potter)
[© 2020 Thomson Reuters. All rights
reserved.] Copyright 2020 Reuters. All rights reserved. This material may not be published,
broadcast, rewritten or redistributed.
Thompson Reuters is solely responsible for this content. |