Europe sets sights on dud antibody tests amid COVID-19 free-for-all
 Send a link to a friend
Send a link to a friend
 [June 11, 2020]
By Francesco Guarascio [June 11, 2020]
By Francesco Guarascio
BRUSSELS (Reuters) - The market for
COVID-19 antibody tests is red-hot. It has ballooned in a matter of
months as hundreds of products flood the world for people who want to
find out whether they've already had the virus.
The problem is, some of them don't work properly.
As a result, European authorities aim to tighten regulation of the new
sector, to weed out tests that give consistently inaccurate results and
crack down on companies that make false claims, three sources familiar
with the plans told Reuters.
Much is on the line, even beyond the potential for fraud.
Governments and companies are relying on these tests to measure how
widely the virus has spread as they rush to get their economies and
employees back to work and avoid a second wave of infections, even if
they do not prove immunity.
False results could undermine that effort.
Many people have also been using kits, also known as serological or
blood tests, at home or for personal checks in clinics.
Since April the number of antibody kits carrying the region's CE mark of
quality doubled to more than 200, according to a list compiled by the EU
Commission, the EU executive.

Some of these kits are unreliable, half a dozen national regulators and
industry sources across Europe told Reuters. A dozen tests have been
subject to regulators' warnings for mis-selling, including in Spain and
Sweden.
At least nine of them are no longer allowed to be sold in the United
States, according to a Reuters analysis of public data from the Food and
Drug Administration, which clamped down on the sector last month.
The EU Commission is now looking at changing the self-certification
regime that allows test-makers to label their products with the CE mark
themselves, an EU official and two European regulatory sources told
Reuters, declining to be named as the plans have not been made public.
Among changes being examined, companies could be required to have tests
reviewed by independent watchdogs before placing the CE mark on them,
the sources said.
That would mark a significant toughening of the current regime, whereby
makers merely self-certify compliance with EU safety rules and
supervisors can subsequently penalise them if their claims turn out to
be false.
Guidance setting out minimum performance criteria for tests could also
be adopted, the two regulatory sources told Reuters. Under current
rules, kits can carry a CE mark regardless of their accuracy.
When asked about the plans, a spokesman for the Commission, the EU
executive, said it was "currently considering the best way forward".
"We are assessing a number of different instruments available together
with member states to see which action is the most appropriate," he
added.
'SPENDING LOTS OF MONEY'
Scientists have not yet definitively proven whether or how long COVID-19
survivors are immune to new infections, even if they developed
antibodies.

Nevertheless, many people are willing to pay for the tests - and many
don't come cheap, selling for anything from a few euros to more than 150
euros in some private clinics in Europe.
It's a potentially lucrative business: testing about 450 million people
in the EU would cost billions of euros based on such prices.
"People might be spending lots of money for nothing," said Pauline
Constant of BEUC, a European consumer organisation.
[to top of second column]
|
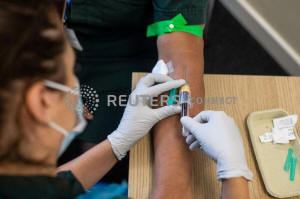
A paramedic takes a blood sample from a medical worker, during an
antibody testing program at the Hollymoor Ambulance Hub of the West
Midlands Ambulance Service, operated by the West Midlands Ambulance
Service NHS Foundation Trust, in Birmingham, Britain June 5, 2020.
Simon Dawson/Pool via REUTERS/File Photo

Severin Schwan, CEO of Swiss drug giant Roche <ROG.S> which has its
own antibody test, sounded an alarm in late April, when he said some
tests on the market were a "disaster". The World Health Organization
also warned that tests on the market were not sufficiently reliable
and could not prove immunity.
Such comments were backed up by a study conducted by the Dutch
regulator who found in May that none of 16 reviewed tests were
trustworthy.
"The test results are not reliable. The percentage of false positive
and false negative results is too high," the study concluded,
without naming the manufacturers.
"Those tests are quite meaningless," said a spokesman for the Dutch
Health and Youth Care Inspectorate, a part of the health ministry
that supervises public health.
Despite the poor results, clinics and labs in the Netherlands are
still allowed to offer these tests.
INDUSTRY BACKS RULE REVIEW
Introducing an independent review of products would bring forward an
EU reform that had been agreed before the coronavirus crisis but was
not due to take effect until 2022.
In the absence of an EU performance standard for tests, France has
already set its own thresholds. About 60 kits have met the country's
requirement of at least 90% of correct positive results, a measure
known as sensitivity, and 98% of correct negative results, so-called
specificity.
Under current EU rules, overseen and enforced by national watchdogs,
manufacturers must seek authorisation before using the CE mark only
for so-called home or self-tests, which are those who can be
performed at home without professional advice.

About a dozen antibody devices have been unlawfully marketed as
CE-marked self-tests without prior authorisation, according to the
Spanish and Swedish regulators.
Low performance is not in itself illegal and has been tolerated in
Europe in the initial phase of the COVID-19 crisis because tests
were rare. But companies cannot exaggerate the accuracy of their
devices.
An official at the Swedish regulator said the body had yet to start
performance checks, while two pharmaceutical industry sources said
such checks were rare in Europe.
There is however a balance to be struck on regulation, according to
industry experts who worry that if new rules are too strict or
onerous they could delay the deployment of tests to conduct
large-scale epidemiological surveys.
But greater scrutiny could help improve the tarnished reputation of
the sector.
MedTechEurope, the trading body for medical technology firms
including Abbott <ABT.N>, Roche <ROG.S> and Siemens Healthineers <SHLG.DE>,
backed plans to review industry regulations.
"We support the intention of these plans, and would welcome the
opportunity to provide the industry's input into this debate on how
to best ensure only well-performing tests are on the market," it
said.
(Reporting by Francesco Guarascio @fraguarascio; Additional
reporting by Emma Pinedo in Madrid, Caroline Humer in New York and
Matthias Blamont in Paris; Editing by Josephine Mason and Pravin
Char)
[© 2020 Thomson Reuters. All rights
reserved.] Copyright 2020 Reuters. All rights reserved. This material may not be published,
broadcast, rewritten or redistributed.
Thompson Reuters is solely responsible for this content. |