U.S. relaxes restriction on abortion pill, allows women to obtain by
mail
 Send a link to a friend
Send a link to a friend
 [December 17, 2021]
NEW YORK (Reuters) - The U.S.
government on Thursday permanently eased some restrictions on a pill
used to terminate early pregnancies, allowing the drug to be sent by
mail rather than requiring it to be dispensed in person. [December 17, 2021]
NEW YORK (Reuters) - The U.S.
government on Thursday permanently eased some restrictions on a pill
used to terminate early pregnancies, allowing the drug to be sent by
mail rather than requiring it to be dispensed in person.
The decision by the Food and Drug Administration comes as the right to
obtain an abortion, established in the 1973 Supreme Court ruling Roe v.
Wade, hangs in the balance.
The medication, generically known as mifepristone, is approved for use
up to 10 weeks of pregnancy and is also sometimes prescribed to treat
women who are having miscarriages.
"The FDA’s decision will come as a tremendous relief for countless
abortion and miscarriage patients," said Georgeanne Usova, senior
legislative counsel at the ACLU.
The restrictions on the pill had been in place since the FDA approved
the drug in 2000 and were lifted temporarily by the government earlier
this year due to the pandemic. That enabled women to consult healthcare
providers by telemedicine and receive the pills by mail. The FDA's
decision makes that temporary change permanent.
As a result of the FDA rule change, many patients will not need to go to
a clinic, medical office or hospital in person to receive the
medication, but can opt to receive the pill through the mail from a
certified prescriber or pharmacy.\

The decision will increase access to medication abortion for women in
remote and rural areas without providers nearby.
Low-income women who face obstacles reaching clinics such as lack of
transportation and inability to take time off work will also gain
greater access to the drug.
However, 19 states including Texas have laws that supersede the FDA
decision by barring telehealth consultations or mailing of abortion
pills. Women in those states would not be able to make use of the rule
change at home but could potentially travel to other states to obtain
medication abortion.
States such as California and New York that have sought to strengthen
access to abortion may make the drug available to women from other
states.
The change is likely to add to the intense U.S. political debate over
abortion. Conservative Supreme Court justices indicated in Dec. 1 oral
arguments over an abortion ban in Mississippi at 15-weeks of pregnancy
that they are open to either gutting Roe or overturning it entirely. A
decision is due by the end of June.
[to top of second column]
|
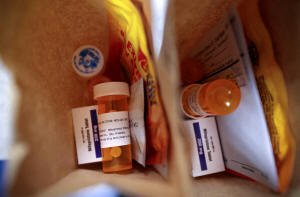
Paper bags containing the medication used for a medical abortion,
follow-up instructions, and heating pads are prepared for patients
who will be having abortions that day at Trust Women clinic in
Oklahoma City, U.S., December 6, 2021. Of the 20 abortions performed
that day, 17 of the patients came from Texas. REUTERS/Evelyn
Hockstein

The Charlotte Lozier Institute and Susan B. Anthony List, which
advocate against abortion, said in a statement that the FDA decision
ignored data on complications and put women at risk.
The groups called on the FDA to restore the in-person dispensing
requirement and add restrictions.
FDA records show that of the 3.7 million women who took Mifeprex,
the branded version of the drug, to terminate a pregnancy between
September 2000 and December 2018, 24 died from complications.
SOME RESTRICTIONS REMAIN
The FDA left in place some restrictions, such as the need to use a
certified pharmacy and requiring the prescribers to be certified.
The ACLU said it was "disappointing that the FDA fell short of
repealing all of its medically unnecessary restrictions on
mifepristone and these remaining obstacles should also be lifted.”
The organization sued the U.S. government on behalf of a
Hawaii doctor and several professional health care associations in
2017 challenging the restrictions that it said limited access to
medication abortion.
Medication abortion involves two drugs, taken over a day or two. The
first, mifepristone, blocks the pregnancy-sustaining hormone
progesterone. The second, misoprostol, induces uterine contractions.
(Reporting by Caroline Humer and Ahmed Aboulenein; additional
reporting by Lawrence Hurley; Editing by Cynthia Osterman)
[© 2021 Thomson Reuters. All rights
reserved.] Copyright 2021 Reuters. All rights reserved. This material may not be published,
broadcast, rewritten or redistributed.
Thompson Reuters is solely responsible for this content.
 |