University of Illinois receives FDA approval for saliva-based COVID-19
test
 Send a link to a friend
Send a link to a friend
[March 02, 2021]
By GRACE BARBIC
Capitol News Illinois
gbarbic@capitolnewsillinois.com
 The University of Illinois System received
Emergency Use Authorization from the U.S. Food and Drug Administration
on Monday for its saliva-based COVID-19 test, as the statewide seven-day
rolling positivity rate reached 2.4 percent. The University of Illinois System received
Emergency Use Authorization from the U.S. Food and Drug Administration
on Monday for its saliva-based COVID-19 test, as the statewide seven-day
rolling positivity rate reached 2.4 percent.
The FDA approval allows for the covidSHIELD test to expand beyond the U
of I System. The saliva-based COVID-19 test has been administered more
than 1.5 million times at universities in Urbana-Champaign, Chicago and
Springfield since it launched in 2020, according to a news release.
Gov. JB Pritzker says he will be dedicating $20 million in federal CARES
Act funding to provide one million of the saliva-based tests to
Illinois’ 12 public universities and 48 community colleges.
According to Pritzker, widespread testing remains critical to combating
the ongoing pandemic. An agreement between the Illinois Department of
Public Health and the U of I System allows for federal funds to be put
toward providing the tests.

“My administration has been proud to work hand in hand with U of I since
the earliest days of this development, which has had an enormously
positive effect on keeping COVID-19 at bay in the U of I System, and
we’re wasting no time in deploying this technology throughout the
state,” Pritzker said in a news release.
The testing process was developed by a team of researchers at UIUC.
The samples are tested at a covidSHIELD lab and individuals receive
results within 24 hours. The test uses a genetic material contained in
SARS-CoV-2 virus to determine if the virus is present or not in the
salvia.
“The University of Illinois has been a national leader in innovation for
decades, and the campus’ groundbreaking work to develop rapid,
saliva-based COVID-19 testing is but the latest example of that
tradition,” Pritzker said.
This comes as the FDA also granted Emergency Use Authorization to a
third COVID-19 vaccine, developed by Johnson & Johnson, over the
weekend.
[to top of second column]
|
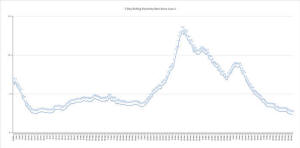
The graph shows the rolling, 7-day positivity rate
for tests completed starting on June 1. Illinois Department of
Public Health data was used to calculate the averages. (Credit:
Jerry Nowicki of Capitol News Illinois)

Vaccine distribution centers could start to see the new single dose
vaccine as early as Tuesday, USA Today reports. White House
officials announced 3.9 million doses could be expected by Tuesday
and a total of 20 million doses could be sent to states throughout
the remainder of the month.
Illinois has received more than 3 million doses of the Pfizer-BioNTech
and Moderna COVID-19 vaccines as of Monday. A total of 2.7 million
doses have been delivered to providers in the state, with an
additional 443,700 doses allocated to the federal government’s
Pharmacy Partnership Program for long-term care facilities.
On Sunday, 50,897 doses of the vaccine were administered in a single
day. A total of 2.7 million vaccines have been administered across
the state, including 319,393 for long-term care facilities, as of
Sunday night.
About 6.5 percent of the state’s population has received two doses
of the COVID-19 vaccine.
The seven-day rolling average of vaccines administered daily is
77,876 doses.
The statewide seven-day rolling positivity rate reached 2.4 percent,
a rate not seen since June 23.
IDPH reported 1,143 new confirmed and probable cases of COVID-19
Monday of 42,234 test results reported, including 20 additional
deaths.
As of Sunday night, 1,288 COVID-19 patients were reported to be
hospitalized. Of those, 308 patients were in intensive care unit
beds and 148 were reported to be on ventilators.
IDPH is currently reporting a total of 1,187,839 cases of more than
18 million total test results reported, including 20,536 total
virus-related deaths since the pandemic started.
Capitol News Illinois is a nonprofit, nonpartisan
news service covering state government and distributed to more than
400 newspapers statewide. It is funded primarily by the Illinois
Press Foundation and the Robert R. McCormick Foundation. |