 Pfizer,
BioNTech seek U.S. COVID-19 vaccine clearance for children 5-11 Pfizer,
BioNTech seek U.S. COVID-19 vaccine clearance for children 5-11
 Send a link to a friend
Send a link to a friend
[October 08, 2021]
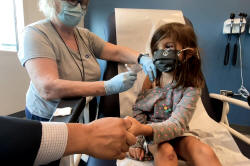
WASHINGTON (Reuters) -Pfizer Inc and
BioNTech SE have asked U.S. regulators to authorize emergency use of
their COVID-19 vaccine for children ages 5 to 11, a group for whom no
shot is currently allowed, Pfizer said on Thursday.
|
|
 The U.S. Food and Drug Administration has set a date of Oct. 26 for
its panel of outside advisers to meet and discuss the application,
making it possible for children in this age group - numbering around
28 million - to begin receiving the two-dose Pfizer/BioNTech vaccine
shortly afterward. The U.S. Food and Drug Administration has set a date of Oct. 26 for
its panel of outside advisers to meet and discuss the application,
making it possible for children in this age group - numbering around
28 million - to begin receiving the two-dose Pfizer/BioNTech vaccine
shortly afterward.
"With new cases in children in the U.S. continuing to be at a high
level, this submission is an important step in our ongoing effort
against #COVID19," Pfizer wrote on Twitter.
The vaccine already has won U.S. emergency use authorization in
teens ages 12 to 15 and is fully approved by regulators for people
ages 16 and up.
The Pfizer/BioNTech vaccine is one of three in use in the United
States, along with the two-dose Moderna vaccine and the single-dose
Johnson & Johnson version, neither of which has won full regulatory
approval for any age group.
A rapid authorization of the Pfizer/BioNTech vaccine in young kids
could help mitigate a potential surge of cases in the coming weeks
and months, with schools open nationwide and colder weather driving
activities indoors. If given regulatory authorization, the two-dose
Pfizer/BioNTech vaccine would become the first COVID-19 shot made
available to children 5 to 11 in the United States.

The Pfizer/BioNTech vaccine has been shown to induce a strong immune
response in 5 to 11 year olds in a 2,268-participant clinical trial,
the companies said on Sept. 20.
The two drugmakers are also testing the vaccine in children ages 2
to 5 years old and children ages 6 months to 2 years, with data
expected in the fourth quarter.
[to top of second column] |
 The vaccine could be ready for
roll out as early as November pending approval
from federal regulatory health agencies, White
House COVID-19 response coordinator Jeffrey
Zients said on CNN.
Once the authorization is granted, Zients said:
"We are ready. We have the supply. We're working
with states to set up convenient locations for
parents and kids to get vaccinated including
pediatricians' offices and community sites."

The United States leads the world in COVID-19
cases and deaths.
Children currently make up about 27% of all U.S.
coronavirus cases and an increasing percentage
of hospitalizations, according to the American
Academy of Pediatrics. That reflects the high
contagiousness of the coronavirus Delta variant
among unvaccinated people.
While children are less susceptible to severe
COVID-19, they can spread the virus to others,
including vulnerable populations more at risk of
severe illness.
A Pfizer spokesperson said the application to
the FDA has been completed.
(Reporting by Susan Heavey in Washington and
Manas Mishra and Manojna Maddipatla in Bengaluru;
Editing by Will Dunham, Timothy Heritage and
Saumyadeb Chakrabarty)
[© 2021 Thomson Reuters. All rights
reserved.] Copyright 2021 Reuters. All rights reserved. This material may not be published,
broadcast, rewritten or redistributed.
Thompson Reuters is solely responsible for this content |