Britain's health regulator backs COVID vaccine for infants from six
months
 Send a link to a friend
Send a link to a friend
 [December 06, 2022]
LONDON (Reuters) -Britain's health regulator on Tuesday
authorised a COVID-19 vaccine for infants as young as six months,
opening the door for vaccinating the country's youngest children once
the UK's Joint Committee on Vaccination and Immunisation (JCVI) agrees. [December 06, 2022]
LONDON (Reuters) -Britain's health regulator on Tuesday
authorised a COVID-19 vaccine for infants as young as six months,
opening the door for vaccinating the country's youngest children once
the UK's Joint Committee on Vaccination and Immunisation (JCVI) agrees.
The Medicines and Healthcare products Regulatory Agency (MHRA)
authorised the vaccine - made by Pfizer and BioNTech - for children aged
six months to four years old, after it was deemed safe and effective
based on an ongoing clinical trial involving 4,526 participants.
Whether the vaccine is eventually deployed in this age group depends on
a recommendation from the JCVI, which advises UK health departments on
which shots should be used as part of the national vaccination programme.
The vaccine is tailored for use in this age group - it is a lower dose
version than the one used in children aged five to 11 years. It is given
as three injections in the upper arm, with the first two doses given
three weeks apart, followed by a third dose administered at least two
months after the second dose.
[to top of second column]
|
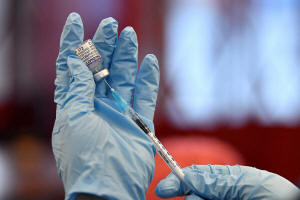
Nurse Christina McCavana prepares the
vials of the Pfizer coronavirus disease (COVID-19) vaccine for use
at a pop-up vaccination clinic in the Central Fire Station in
Belfast, Northern Ireland, December 4, 2021. REUTERS/Clodagh
Kilcoyne/File Photo
 U.S. officials rolled out this
version of the Pfizer-BioNTech shot for the same age group earlier
this year. Months ago, EU regulators also endorsed the use of COVID
vaccines made by Pfizer-BioNTech and Moderna for under-fives.
(Reporting by Natalie Grover in London; Editing by Jan Harvey,
Alexandra Hudson)
[© 2022 Thomson Reuters. All rights
reserved.] This material may not be published,
broadcast, rewritten or redistributed.
Thompson Reuters is solely responsible for this content. |