U.S. CDC backs full approval of Moderna's COVID-19 vaccine
 Send a link to a friend
Send a link to a friend
 [February 05, 2022]
(Reuters) - The director of the U.S.
Centers for Disease Control and Prevention (CDC) signed off on the U.S.
Food and Drug Administration's full approval of Moderna Inc's COVID-19
vaccine in those aged 18 and over, the agency said on Friday. [February 05, 2022]
(Reuters) - The director of the U.S.
Centers for Disease Control and Prevention (CDC) signed off on the U.S.
Food and Drug Administration's full approval of Moderna Inc's COVID-19
vaccine in those aged 18 and over, the agency said on Friday.
The vaccine has been in use under the U.S Food and Drug Administration's
emergency use authorization since December 2020, and is now the second
fully approved vaccine for COVID-19 in the United States.
Earlier on Friday, a CDC panel voted unanimously to recommend the
vaccine's use, after the FDA granted full approval of the shot on
Monday.
While the FDA approves vaccines, the CDC needs to sign off on how they
will be implemented in the United States. CDC Director Rochelle
Walensky's green light is the final formality of the approval process.

[to top of second column]
|
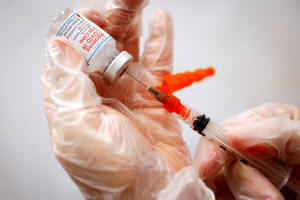
A healthcare worker prepares a syringe with the Moderna COVID-19
vaccine at a pop-up vaccination site operated by SOMOS Community
Care during the COVID-19 pandemic in Manhattan in New York City, New
York, U.S., January 29, 2021. REUTERS/Mike Segar
 The vaccine will now be sold under
the brand name as Spikevax.
The COVID-19 vaccine from Pfizer Inc and BioNTech SE received full
approval for those aged 16 and over in August.
Roughly 75 million people in the United States have been
fully-vaccinated with Moderna's shot.
(Reporting by Manas Mishra in Bengaluru and Michael Erman in New
Jersey; Editing by Bill Berkrot and Anil D'Silva)
[© 2022 Thomson Reuters. All rights
reserved.] This material may not be published,
broadcast, rewritten or redistributed.
Thompson Reuters is solely responsible for this content. |