U.S. orders 3.2 million doses of Novavax
COVID vaccine
 Send a link to a friend
Send a link to a friend
 [July 12, 2022]
(Reuters) -The U.S. government will
get 3.2 million doses of COVID-19 vaccine developed by Novavax Inc once
the shot has been authorized by the regulators, the Department of Health
and Human Services (HHS) and the company said on Monday. [July 12, 2022]
(Reuters) -The U.S. government will
get 3.2 million doses of COVID-19 vaccine developed by Novavax Inc once
the shot has been authorized by the regulators, the Department of Health
and Human Services (HHS) and the company said on Monday.
The shot will be made available for free in the country after it gets
authorization by the U.S. Food and Drug Administration (FDA) for
emergency use and the Centers for Disease Control and Prevention's (CDC)
recommendation.
Advisers to the FDA last month voted to recommend that the agency
authorize the shot for use in adults.
Novavax is expected to complete all necessary quality testing in the
next few weeks, which would support final release of the shots, HHS said
in a statement on Monday.
The company said last month the initial doses of the vaccine will be
manufactured by its partner Serum Institute of India.

[to top of second column]
|
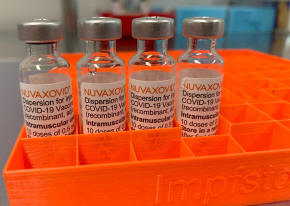
Four vials with the "Nuvaxovid" COVID-19 vaccine from Novavax are
pictured in Saabruecken, Germany, February 26, 2022. REUTERS/Frank
Simon/File Photo
 Novavax's shot, which is already
available in over 40 countries, is a more traditional type of
vaccine employing technology that has been used for decades to
combat diseases like influenza.
Maryland-based Novavax is hoping to gain a foothold within the
roughly 27 million U.S. adults who are yet to be vaccinated,
particularly those who do not want to receive a vaccine like the
Pfizer/BioNTech or Moderna Inc shots, which are based on the
groundbreaking messenger RNA (mRNA) technology.
(Reporting by Ankur Banerjee in Bengaluru; Editing by Shinjini
Ganguli)
[© 2022 Thomson Reuters. All rights
reserved.] This material may not be published,
broadcast, rewritten or redistributed.
Thompson Reuters is solely responsible for this content. |