Bavarian Nordic monkeypox vaccine gets preliminary nod from EU drug
regulator
 Send a link to a friend
Send a link to a friend
 [July 22, 2022]
COPENHAGEN (Reuters) - Danish
biotechnology company Bavarian Nordic said on Friday the European
Union's drug regulator had recommended its Imvanex vaccine be approved
to also include protection against monkeypox on its label. [July 22, 2022]
COPENHAGEN (Reuters) - Danish
biotechnology company Bavarian Nordic said on Friday the European
Union's drug regulator had recommended its Imvanex vaccine be approved
to also include protection against monkeypox on its label.
The European Medicines Agency (EMA) had "adopted a positive opinion
recommending that the marketing authorisation for the company's smallpox
vaccine, IMVANEX, is extended to include protecting people from
monkeypox disease," Bavarian said.
Bavarian's vaccine, the only one to have won approval for the prevention
of monkeypox disease in the United States and Canada, has in the EU so
far only been approved to treat smallpox.
But the company has supplied the vaccine to several EU countries during
the current monkeypox outbreak for what is known as "off-label" use.

[to top of second column]
|
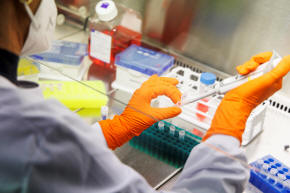
An employee of the vaccine company Bavarian Nordic works in a
laboratory of the company in Martinsried near Munich, Germany, May
24, 2022. The company, headquartered in Denmark, is the only one in
the world to have approval for a smallpox vaccine called Jynneos in
the U.S. and Imvanex in Europe, which is also effective against
monkeypox. REUTERS/Lukas Barth
 "The extension of the label will
help to improve access to the vaccine throughout Europe and
strengthen the future preparedness against monkeypox," Bavarian CEO
Paul Chaplin said in a statement.
The recommendation from the EMA will be referred to the European
Commission for final approval shortly, the company said.
(Reporting by Nikolaj Skydsgaard; editing by Jason Neely)
[© 2022 Thomson Reuters. All rights
reserved.] This material may not be published,
broadcast, rewritten or redistributed.
Thompson Reuters is solely responsible for this content. |