U.S. FDA opens way to COVID vaccines for kids under 5, CDC up next
 Send a link to a friend
Send a link to a friend
 [June 18, 2022]
By Manas Mishra and Michael Erman [June 18, 2022]
By Manas Mishra and Michael Erman
(Reuters) -The U.S. Food and Drug
Administration on Friday authorized two COVID-19 vaccines for children
under 5, opening the door to vaccinating millions of the country's
youngest children once the Centers for Disease Control and Prevention
agrees.
The FDA authorized Pfizer-BioNTech's COVID-19 vaccine for children aged
6 months to 4 years and Moderna Inc's shot for those 6 months to 17
years. Pfizer's is already authorized for those over the age of 5.
The vaccines could be rolled out to the under-5 age groups as early as
next week, White House officials have said. The CDC needs to make its
recommendations on how the shots should be administered before a
vaccination campaign can begin in earnest.
"We will begin shipping millions of vaccine doses for kids to thousands
of locations parents know and trust—including pediatricians’ offices,
children’s hospitals, and pharmacies," Biden said in a statement on
Friday.
"As doses are delivered, parents will be able to start scheduling
vaccinations for their youngest kids as early as next week, with
appointments ramping up over the coming days and weeks."

A panel of outside advisers began meeting on Friday to consider a
recommendation to the CDC on the shots for those under 5 years old and
will vote on Saturday, likely followed by the CDC itself greenlighting
the recommendation.
While many parents in the United States are eager to vaccinate their
children, it is unclear how strong demand will be for the shots. The
Pfizer-BioNTech vaccine was authorized for children aged 5 to 11 in
October, but only about 29% of that group is fully vaccinated so far,
federal data shows.
[to top of second column]
|
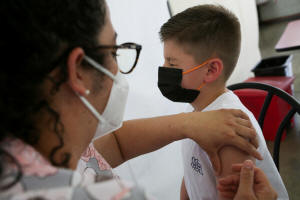
A child is administered a dose of the Pfizer-BioNTech coronavirus
disease (COVID-19) pediatric vaccine, in San Jose, Costa Rica
February 23, 2022. REUTERS/Mayela Lopez

COVID-19 is generally milder in children. Still,
since March 2020 it has been the fifth leading cause of deaths in
children aged 1-4 and the fourth leading cause of death in children
younger than 1, according to the CDC.
"Although the number of deaths in children is small by adult
standards, any death of a child is tragic and should be prevented if
possible," FDA Commissioner Robert Califf said at a press conference
on Friday. "By vaccinating our youngest children, we hope to prevent
the most devastating consequences of COVID."
CVS Health Corp, Walmart Inc and Rite Aid Corp plan to provide
COVID-19 vaccinations to young children in the United States if the
vaccines are authorized, the companies said on Thursday.
Public health officials and experts say that even though a large
portion of small children were infected during the winter surge due
to the Omicron variant of the coronavirus, natural immunity wanes
over time and vaccinations should help prevent hospitalizations and
deaths when cases rise again.
The CDC advisers will meet again next week to consider whether to
back the use of Moderna for older children, aged 6-17. There has
been some concern about the rate of rare cases of heart inflammation
in young men from the Moderna vaccine, and the advisers are expected
to consider that data.
Moderna shares rose 5% while Pfizer shares were down 2% in afternoon
trading.
(Reporting by Manas Mishra in Bengaluru; Editing by Sriraj Kalluvila,
Peter Henderson, Mark Porter and Leslie Adler)
[© 2022 Thomson Reuters. All rights
reserved.]
This material may not be published,
broadcast, rewritten or redistributed.
Thompson Reuters is solely responsible for this content. |