U.S. sends experimental antibody, antiviral drug to Uganda for Ebola
outbreak
 Send a link to a friend
Send a link to a friend
 [October 19, 2022]
By Julie Steenhuysen [October 19, 2022]
By Julie Steenhuysen
CHICAGO (Reuters) - The United States sent
Gilead Sciences' remdesivir and Mapp Biopharmaceutical Inc's
experimental Ebola antibody drug MBP134 to Uganda last week to help
safeguard healthcare workers responding to an outbreak that has infected
60 people and killed 44, U.S. government sources told Reuters.
There are currently no proven vaccines or treatments for the Sudan
species of Ebola, one of four known Ebola viruses to cause hemorrhagic
fever in humans. The outbreak confirmed by the Ugandan health ministry
on Sept. 20 is the largest of the Sudan species since 2000.
Uganda health minister Jane Ruth Aceng disclosed the U.S. shipments at a
meeting of African region health officials last week in Kampala and said
remdesivir, which has been widely used as a COVID-19 treatment, and an
undisclosed monoclonal antibody had been given to healthcare workers.
Providing treatment that protects the lives of healthcare workers could
be central to containing the outbreak, said Joel Montgomery, the U.S.
Centers for Disease Control and Preventionís chief of the viral special
pathogens branch and incident manager for the outbreak.

"If healthcare workers start to fall ill and die, it's going to
negatively impact the response," said Montgomery, who had just returned
from a trip to Uganda.
For instance, healthcare workers may be reluctant to assist in the
response, he said in a phone interview.
The World Health Organization said in a statement the agency is working
with partners in Uganda to set up the infrastructure for a clinical
trial and is supporting use of the untested antivirals and monoclonal
antibodies and will collect data on their efficacy.
A large outbreak of the Zaire species of Ebola in West Africa from
2014-2016 led to effective vaccines and treatment, but there are no
proven treatments or vaccines for the Sudan species.
[to top of second column]
|
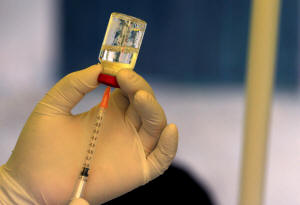
A Ugandan health worker prepares to
administer the ebola vaccine to a man in Kirembo village, near the
border with the Democratic Republic of Congo in Kasese district,
Uganda, June 16, 2019. REUTERS/James Akena/
 San Diego-based Mapp
Biopharmaceutical received a $110 million contract from the U.S.
government's Biomedical Advanced Research and Development Authority
(BARDA) on Oct. 4 for advanced development and potential purchases
of MBP134, a combination of monoclonal antibodies.
Gilead did not immediately respond to a request for comment.
A study of MBP134 and remdesivir in non-human primates showed that
either drug given individually rescued 20% of animals infected with
the Sudan species of Ebola, but when given in combination, 80% of
infected animals survived.
MBP134 is currently being tested in early safety trials in healthy
human volunteers, Mapp President Larry Zeitlin said in an email. All
participants have completed the study, and the data are currently
being analyzed. Overall, MBP134 was well tolerated, he said.
Zeitlin said when requested, the company does provide its drug for
free for compassionate use, pending regulatory and ethics approvals.
He declined to say how many doses the company provided.
(Reporting by Julie Steenhuysen; Additional reporting by Jennifer
Rigby in London and Elias Biryabarema in Kampala; editing by
Caroline Humer and Bill Berkrot)
[© 2022 Thomson Reuters. All rights
reserved.] This material may not be published,
broadcast, rewritten or redistributed.
Thompson Reuters is solely responsible for this content.
 |