Artificial heart maker CARMAT gets regulatory approvals to resume sales
 Send a link to a friend
Send a link to a friend
 [October 25, 2022]
PARIS (Reuters) - French medical
devices company CARMAT said it had received the necessary regulatory
approvals to resume commercial implants of its Aeson artificial hearts
product and would resume sales soon. [October 25, 2022]
PARIS (Reuters) - French medical
devices company CARMAT said it had received the necessary regulatory
approvals to resume commercial implants of its Aeson artificial hearts
product and would resume sales soon.
"We will be resuming implants very soon and moving forward at a measured
pace as we continue building our inventory of implantable prostheses,
and ensure all patients are suitably monitored," Chief Executive
Stéphane Piat said in a statement.
[to top of second column]
|
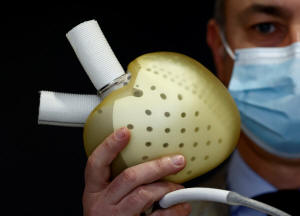
Chief Executive Officer of French
artificial heart manufacturer Carmat, Stephane Piat, poses holding
an artificial heart during an interview with Reuters in Velizy, near
Paris, January 11, 2021. REUTERS/Christian Hartmann/File Photo
 (Reporting by Sudip Kar-Gupta; Editing by Clarence Fernandez)
[© 2022 Thomson Reuters. All rights
reserved.] This material may not be published,
broadcast, rewritten or redistributed.
Thompson Reuters is solely responsible for this content. |