White House plans support for drugstores, pharma in abortion pill battle
-sources
 Send a link to a friend
Send a link to a friend
 [April 10, 2023]
By Nandita Bose [April 10, 2023]
By Nandita Bose
WASHINGTON (Reuters) -The White House is planning to re-up discussions
with abortion pill manufacturers and U.S. pharmacy chains on ways to
push back against efforts to ban mifepristone, two sources with
knowledge of the matter said, as it appeals a Texas court ruling
suspending the approval of the drug.
U.S. District Judge Matthew Kacsmaryk in Amarillo, Texas, on Friday
suspended approval of mifepristone, which will essentially make sales of
the pill illegal in the U.S., while a legal challenge proceeds. A
conflicting Washington state ruling on Friday blocks changes to pill
sales in 17 states.
In January, the Food and Drug Administration made a regulatory change
that made it possible for retail pharmacies to offer abortion pills in
the country for the first time, but more than a dozen states have passed
laws limiting such sales.
There are no retail pharmacies that are currently certified to dispense
mifepristone and many are going through the certification process.
"We are discussing ways to offer them legal support," one of the sources
said of manufacturers and retail pharmacies.

Options being discussed include having the U.S. Department of Justice
back any legal challenges brought against manufacturers and pharmacies,
and providing legal advice on how they can continue dispensing the
pills, the sources said. The DOJ is separately seeking an emergency stay
of the Texas order.
The White House does not direct the DOJ's litigation strategy, a senior
administration official said. The official also said the Justice
Department does not provide legal advice to private entities. There is a
separate volunteer process that gets private attorneys and law firms to
do legal work on different issues that the agency oversees.
It was not immediately clear which companies will be involved in these
discussions. The White House declined comment.
Major U.S. manufacturers of abortion pills include GenBioPro Inc and
Danco Laboratories. Pharmacy chains dispensing such pills include
Walgreens Boots Alliance Inc , CVS Health Corp and Rite Aid Corp.
Walgreens said in March it would not dispense abortion pills in the 20
states where it risked breaking the law.
On Sunday, Walgreens declined comment. The other companies did not
respond to a request for comment on any discussions with the White
House.
The administration official said the White House has "absolutely
previously connected with both pharmacies and manufacturers but we have
not had any discussions on this since Friday when the decision came
out."
Any potential future conversations will not include decisions that
impact "how the FDA will operate or is operating," the official said.

The White House's Gender Policy Council, Inter Governmental Affairs and
the vice president's office have been holding strategy calls for nearly
two months on how to make medical abortion available after the Texas
judge ruled, anticipating Friday's outcome.
[to top of second column]
|
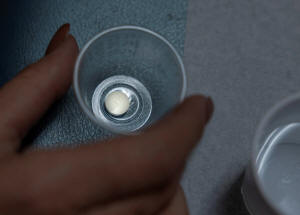
A patient prepares to take mifepristone,
the first pill given in a medical abortion, at Women's Reproductive
Clinic of New Mexico in Santa Teresa, U.S., January 13, 2023.
REUTERS/Evelyn Hockstein
 Discussions between the Biden
administration and pill manufacturers and pharmacies over the issue
have been ongoing for months, sources said, but Friday's decision
brings fresh urgency.
RELIEF FROM WASHINGTON STATE
A flurry of White House strategy calls on Friday and Saturday also
focused on "immediate, short-term" relief offered by the conflicting
order from Washington state , three sources said.
Minutes after Texas judge Kacsmaryk's order, U.S. District Judge
Thomas Rice in Spokane, Washington, an Obama appointee, ordered the
FDA not to make any changes to mifepristone access in some
Democrat-led states.
The administration believes that the Washington ruling gives it more
time to respond legally to Texas's final decision. It could
"expedite review of Texas, encourage an immediate stay on it and
puts a huge question mark over it," one of the sources said.
Politically, the sources said, it makes it easier for the White
House to make its case to the public that the FDA approval of the
drug was accurate, mobilize activists and supporters to turn the
issue into one that resonates with voters ahead of the 2024
presidential elections.
"There's a lot of legal analysis that needs to get done...about how
these two orders interact," the senior administration official said.
President Joe Biden and Vice President Kamala Harris said on Friday
the administration will fight the Texas ruling.
"We're going to fight it. The Attorney General has announced @TheJusticeDept
will file an appeal and seek an immediate stay of the decision,"
Biden tweeted.

The legal battle is likely to work through multiple levels of
appeals courts over a period of months or years before it is
resolved.
The administration is seeking an emergency stay of Kacsmaryk's order
from the New Orleans-based 5th U.S. Circuit Court of Appeals.
The path forward discussed during the White House strategy calls
touched on how the DOJ will wait for a decision from the 5th
Circuit, which has a conservative reputation, the sources said.
If it does not stay the ruling, the department will seek an
expedited review by the Supreme Court, they said.
(Reporting by Nandita Bose in Washington; Editing by Heather
Timmons, Marguerita Choy and Diane Craft)
[© 2023 Thomson Reuters. All rights
reserved.]This material may not be published,
broadcast, rewritten or redistributed.
Thompson Reuters is solely responsible for this content. |