U.S. Supreme Court's Alito temporarily blocks abortion pill curbs
 Send a link to a friend
Send a link to a friend
 [April 15, 2023]
By Andrew Chung [April 15, 2023]
By Andrew Chung
WASHINGTON (Reuters) -U.S. Supreme Court Justice Samuel Alito on Friday
temporarily halted lower court rulings that set limits on access to the
abortion pill mifepristone, giving the nation's top judicial body time
to weigh a bid by President Joe Biden's administration to defend the
drug amid a challenge by anti-abortion groups.
The action by the conservative justice, who handles emergency matters
arising from a group of states including Texas, freezes the litigation
and maintains the current availability of mifepristone pending a further
order from himself or the entire court.
The U.S. Justice Department and Danco Laboratories, the pill's
manufacturer, filed emergency requests earlier on Friday asking the
justices to freeze an April 7 preliminary injunction by Texas-based U.S.
District Judge Matthew Kacsmaryk that would greatly restrict
mifepristone's distribution while litigation contesting its federal
regulatory approval proceeds.
Alito acted just hours before the restrictions were due to have taken
effect. He directed the challengers to respond by Tuesday to the
requests by the Justice Department and Danco, while delaying the
restrictions from taking effect until 11:59 p.m. EDT (0359 GMT) on
Wednesday. The court would be expected to issue another order on the
issue by that time.

The administration is seeking to defend the availability of mifepristone
in the face of mounting abortion bans and restrictions enacted by
Republican-led states since the Supreme Court in June 2022 overturned
the landmark 1973 Roe v. Wade decision that had legalized the procedure
nationwide. Alito authored that ruling for the court, which has a 6-3
conservative majority.
The administration and Danco told the justices in their filings that
mifepristone might not be available for months if the restrictions were
allowed to take effect.
Mifepristone, approved by the U.S. Food and Drug Administration (FDA) in
2000, is used in combination with another drug called misoprostol to
perform medication abortions, which account for more than half of all
U.S. abortions. The FDA is the U.S. agency that signs off on the safety
of food products, drugs and medical devices.
The Justice Department said the lower court orders issued in the past
week limiting mifepristone's availability would have "sweeping
consequences" for women who need access to it and the FDA's scientific
judgment authority over drug safety.
Danco said it may be forced to halt operations in the face of regulatory
uncertainty.
Current drug labels for mifepristone do not account for the new limits
and would have to be adjusted, a process that could last months, the
Justice Department and Danco said in their filings. The generic version
of mifepristone would also lose its approval, the department said.
"The resulting disruption would deny women lawful access to a drug FDA
deemed a safe and effective alternative to invasive surgical abortion,"
the department told the justices.

White House spokesperson Karine Jean-Pierre said in a statement the
Biden administration would continue to stand by the FDA's decisions and
that "the stakes of this fight could not be higher in the face of
ongoing attacks on women's health."
[to top of second column]
|
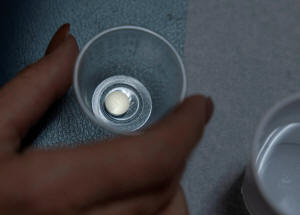
A patient prepares to take mifepristone,
the first pill given in a medical abortion, at Women's Reproductive
Clinic of New Mexico in Santa Teresa, U.S., January 13, 2023.
REUTERS/Evelyn Hockstein/File Photo
 In a case that could undercut the
FDA's authority to decide on the safety of drugs, the New
Orleans-based 5th U.S. Circuit Court of Appeals on Wednesday
declined the administration's request to block Kacsmaryk's
restrictions. The 5th Circuit halted another part of Kacsmaryk's
order that would have suspended FDA approval of the drug,
effectively pulling it off the market.
Kacsmaryk's decision conflicted with an order also issued April 7 in
a separate case from Washington state directing the FDA to keep
mifepristone available in 17 states and the District of Columbia.
Anti-abortion groups led by the recently formed Alliance for
Hippocratic Medicine and four anti-abortion doctors sued the FDA in
November seeking to reverse approval of mifepristone.
'FUNDAMENTAL ERRORS'
"To the government's knowledge, this is the first time any court has
abrogated FDA's conditions on a drug's approval based on a
disagreement with the agency's judgment about safety - much less
done so after those conditions have been in effect for years. And
the lower courts reached that unprecedented result only through a
series of fundamental errors," the Justice Department said in its
filing.
The restrictions set by the lower courts would restore curbs on
mifepristone that had been lifted since 2016 as the FDA steadily
expanded access. These revived restrictions would include requiring
three in-person doctor visits to obtain mifepristone and limiting
its use to the first seven weeks of pregnancy, down from the current
10.

Some 61% of Americans, including 51% of Republicans, oppose efforts
to restrict access to abortion pills, according to a Reuters/Ipsos
poll concluded on Wednesday. Just 37% of respondents said they
trusted the Supreme Court to act impartially in abortion-related
cases.
The Justice Department has said the anti-abortion plaintiffs have no
basis for second-guessing FDA scientific judgment and that when used
as directed, adverse effects of mifepristone are exceedingly rare
"just as they are for many common drugs like ibuprofen." The
challengers have called the restrictions critical safeguards to a
medication they consider dangerous.
Since last year's Supreme Court decision, 12 U.S. states have put in
place outright bans while many others prohibit abortion after a
certain length of pregnancy. The latest Republican-led move came in
Florida, where Governor Ron DeSantis on Thursday signed a new law
that bans most abortions after six weeks of pregnancy.
(Reporting by Andrew Chung in New York; Additional reporting by
Trevor Hunnicutt; Editing by Will Dunham)
[© 2023 Thomson Reuters. All rights
reserved.]This material may not be published,
broadcast, rewritten or redistributed.
Thompson Reuters is solely responsible for this content. |