New scheme aims to get vaccines to outbreaks faster - Gavi
 Send a link to a friend
Send a link to a friend
 [June 28, 2023]
By Jennifer Rigby [June 28, 2023]
By Jennifer Rigby
LONDON (Reuters) - Three global health bodies are teaming up to
investigate stockpiling experimental vaccines for rare infectious
diseases so the shots can be tested more quickly when outbreaks happen,
a top official from vaccine alliance Gavi told Reuters.
The initiative will be led by Gavi, the World Health Organization (WHO)
and the Coalition for Epidemic Preparedness Innovations (CEPI), and is
likely to be announced later on Tuesday after Gavi’s board approved it
at a meeting earlier in the day.
It will focus initially on Marburg and the Sudan strain of Ebola, after
outbreaks of the two deadly viral haemorrhagic fevers in Africa last
year. There are no existing vaccines or proven treatments available for
either of the infections.
If successful, the scheme – known as the global virtual pooled inventory
(GVPI) – could be a pilot for other deadly diseases and wider pandemic
preparedness, officials said, which are an increasing threat due to
factors like climate change.

In Uganda last year, 55 people died in the Ebola outbreak and there were
142 confirmed cases, according to WHO. Equatorial Guinea and Tanzania
saw 25 confirmed Marburg cases and 18 deaths in total. There were a
further 23 probable cases, all of whom died, in Equatorial Guinea, which
had never experienced Marburg before.
While the governments moved fast with global partners to try to set up
human trials of new vaccines, the outbreaks were halted with other
public health measures, like testing and isolating patients, before
trials could properly begin.
While that speed clearly saved lives, it also meant that the world was
not really any closer to having effective vaccines available to help
tackle future outbreaks, said Aurelia Nguyen, chief programme strategy
officer at Gavi.
[to top of second column]
|
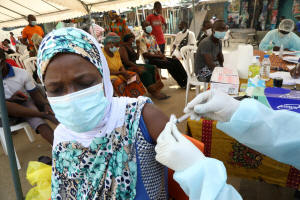
A resident receives a vaccine as the
vaccinations against Ebola continue in Alakro, the slum where the
first case of Ebola was confirmed, in Abidjan, Ivory Coast August
17, 2021. REUTERS/Luc Gnago/File Photo
 “We’ve had the lesson with Ebola
Sudan and with Marburg,” she told Reuters. “Think about a mechanism
where we have the ability to secure some doses of an investigational
vaccine ... in a way that actually gets us ahead of the outbreak.”
The details of the plan are still being worked out, but Nguyen said
it could work with Gavi and partners agreeing deals ahead of time,
where manufacturers commit to providing a certain number of vaccine
doses very quickly when outbreaks begin. A similar model worked for
another Ebola strain, Zaire.
Vaccines for Ebola Sudan are under development by companies and
researchers including Oxford University and the Serum Institute of
India as well as the International Aids Vaccine Institute (IAVI) and
Merck, and the Sabin Institute. The latter is also working on a
Marburg vaccine, among other companies and institutions.
Vaccines would need to have been tested for safety and whether they
induced an immune response in humans - usually phase II trials -
before being included in the stockpile, said Nguyen.
(Reporting by Jennifer Rigby; Editing by Mark Potter)
[© 2023 Thomson Reuters. All rights
reserved.]This material may not be published,
broadcast, rewritten or redistributed.
Thompson Reuters is solely responsible for this content.
 |