UK regulator approves second Alzheimer's drug in months but government
won't pay for it
 Send a link to a friend
Send a link to a friend
 [October 24, 2024]
By MARIA CHENG [October 24, 2024]
By MARIA CHENG
LONDON (AP) — Britain's drug regulator approved the Alzheimer's drug
Kisunla on Wednesday, but the government won't be paying for it after an
independent watchdog agency said the treatment isn't worth the cost to
taxpayers.
It is the second Alzheimer's drug to receive such a mixed reception
within months. In August, the U.K. regulator authorized Leqembi while
the same watchdog agency issued draft guidance recommending against its
purchase for the National Health Service.
In a statement on Wednesday, Britain's Medicines and Healthcare
regulatory Agency said Kisunla “showed some evidence of efficacy in
slowing (Alzheimer's) progression” and approved its use to treat people
in the early stages of the brain-robbing disease. Kisunla, also known as
donanemab, works by removing a sticky protein from the brain believed to
cause Alzheimer’s disease.
Meanwhile, the National Institute for Health and Care Excellence, or
NICE, said more evidence was needed to prove Kisunla's worth — the
drug's maker, Eli Lilly, says a year's worth of treatment is $32,000.
The U.S. Food and Drug Administration authorized Kisunla in July. The
roll-out of its competitor drug Leqembi has been slowed in the U.S. by
spotty insurance coverage, logistical hurdles and financial worries.
NICE said that the cost of administering Kisunla, which requires regular
intravenous infusions and rigorous monitoring for potentially severe
side effects including brain swelling or bleeding, “means it cannot
currently be considered good value for the taxpayer.”
Experts at NICE said they “recognized the importance of new treatment
options” for Alzheimer's and asked Eli Lilly and the National Health
Service “to provide additional information to address areas of
uncertainty in the evidence.”

[to top of second column]
|
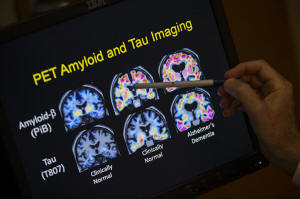
R. Scott Turner, Professor of Neurology and Director of the Memory
Disorder Center at Georgetown University Hospital, points to PET
scan results that are part of a study on Alzheimer's disease at
Georgetown University Hospital, in Washington, May 19, 2015. (AP
Photo/Evan Vucci, File)
 Under Britain's health care system,
most people receive free health care paid for by the government, but
they could get Kisunla if they were to pay for it privately.
“People living with dementia and their loved ones will undoubtedly
be disappointed by the decision not to fund this new treatment,”
said Tara Spires-Jones, director of the Centre for Discovery Brain
Sciences at the University of Edinburgh. “The good news that new
treatments can slow disease even a small amount is helpful," she
said in a statement, adding that new research would ultimately bring
safer and more effective treatments.
Fiona Carragher, chief policy and research officer at the
Alzheimer's Society, said the decision by NICE was “disheartening,”
but noted there were about 20 Alzheimer's drugs being tested in
advanced studies, predicting that more drugs would be submitted for
approval within years.
“In other diseases like cancer, treatments have become more
effective, safer and cheaper over time,” she said. “ We hope to see
similar progress in dementia."
___
The Associated Press Health and Science Department receives support
from the Howard Hughes Medical Institute’s Science and Educational
Media Group. The AP is solely responsible for all content.
All contents © copyright 2024 Associated Press. All rights reserved |