People 50 and older should get pneumococcal vaccine, U.S. health
officials recommend
 Send a link to a friend
Send a link to a friend
 [October 24, 2024]
By MIKE STOBBE [October 24, 2024]
By MIKE STOBBE
NEW YORK (AP) — U.S. health officials on Wednesday recommended that
people 50 and older get a shot against bacteria that can cause pneumonia
and other dangerous illnesses.
The recommendation was made by a scientific advisory panel and then
accepted by the Centers for Disease Control and Prevention. The decision
lowered — from 65 — the minimum recommended age for older adults to get
the shot.
“Now is a great time to get vaccinated against pneumococcal disease in
preparation for the winter respiratory season,” CDC Director Dr. Mandy
Cohen said in a statement Wednesday night.
The advisory committee voted 14-1 to make the change during a meeting
earlier in the day in Atlanta. The guidance is widely heeded by doctors
and prompts health insurers to pay for recommended shots.
Pneumococcal shot recommendations are sometimes called the most
complicated vaccination guidance that the government issues. The CDC
currently recommends shots for children younger than 5 and adults 65 or
older, as long as they have never been vaccinated against pneumococcal
disease. Officials also recommend the shots for children and adults at
increased risk for pneumococcal disease, such as those with diabetes,
chronic liver disease or a weakened immune system.
There are more than 100 known types of pneumococci bacteria, which can
cause serious infections in the lungs and other parts of the body. Each
year, the U.S. sees roughly 30,000 cases of invasive pneumococcal
disease, which includes blood infections, brain and spine inflammation,
and other illnesses. About 30% of cases are among 50- to 64-year-olds.

The first pneumococcal vaccine was licensed in the U.S. in 1977, and
since then pharmaceutical companies have been coming up with newer
versions that target a dozen or more types in a single shot. Different
vaccines have fallen in and out of favor, including Pfizer’s Prevnar 13,
which was once a top-seller but is no longer available.
[to top of second column]
|
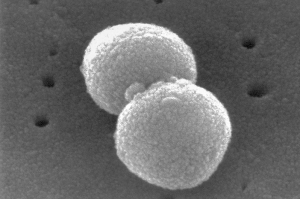
This electron microscopic image provided by the Centers for Disease
Control and prevention depicts two, round-shaped, Gram-positive,
Streptococcus pneumoniae bacteria. (Janice Haney Carr/CDC via AP)
 There are four vaccines now in use.
The U.S. Food and Drug Administration this year approved the newest
— Merck’s Capvaxive, which can cost around $300 a dose and protects
against 21 types, including eight not included in other pneumococcal
vaccines. A Merck spokesperson said it was specifically designed to
help protect against the bacteria types that cause the majority of
severe disease in adults aged 50 and older.
The CDC advisory panel in June recommended the vaccine as an option
for adults at higher risk. At the time, the committee also talked
about the possibility of lowering the age recommendation for older
adults. They noted that illness-causing infections peak at age 55 to
59 in Black Americans — a lower age than what’s seen in white
people. But the committee put off that decision until this week's
meeting.
Some concerns: A booster shot may prove to be necessary, perhaps in
about 15 years. And there are some new vaccines in development that
could force another update to the recommendations.
“Pneumococcal has been a very confusing recommendation for many,
many years and it’s hard to have a new recommendation every two or
three years,” said Dr. Jamie Loehr, chair of the committee’s
pneumococcal working group. He was the only person to vote against
the proposal.
___
The Associated Press Health and Science Department receives support
from the Howard Hughes Medical Institute’s Science and Educational
Media Group. The AP is solely responsible for all content.
All contents © copyright 2024 Associated Press. All rights reserved |