FDA OKs libido-boosting pill for older women who have gone through
menopause
[December 16, 2025]
By MATTHEW PERRONE
WASHINGTON (AP) — U.S. health officials have expanded approval of a
much-debated drug aimed at boosting female libido, saying the once-a-day
pill can now be taken by postmenopausal women up to 65 years old.
The announcement Monday from the Food and Drug Administration broadens
the drug's use to older women who have gone through menopause. The pill,
Addyi, was first approved 10 years ago for premenopausal women who
report emotional stress due to low sex drive.
Addyi, marketed by Sprout Pharmaceuticals, was initially expected to
become a blockbuster drug, filling an important niche in women's health.
But the drug came with unpleasant side effects including dizziness and
nausea, and it carries a safety warning about the dangers of combining
it with alcohol.
The boxed warning cautions that drinking while consuming the pill can
cause dangerously low blood pressure and fainting. If patients have
several drinks, the label recommends waiting a few hours before taking
the drug, or skipping one dose.
Sales of Addyi, which acts on brain chemicals that affect mood and
appetite, fell short of Wall Street's initial expectations. In 2019, the
FDA approved a second drug for low female libido, an on-demand injection
that acts on a different set of neurological chemicals.
Sprout CEO Cindy Eckert said in a statement the approval “reflects a
decade of persistent work with the FDA to fundamentally change how
women’s sexual health is understood and prioritized.” The company, based
in Raleigh, North Carolina, announced the FDA update in a press release
Monday.

[to top of second column]
|
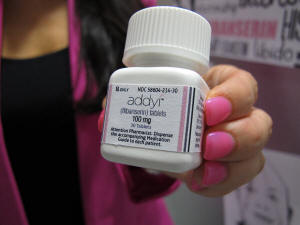
In this Aug. 18, 2015 file photo, Sprout Pharmaceuticals CEO
Cindy Whitehead holds a bottle for the female sex-drive drug Addyi
in Raleigh, N.C. (AP Photo/Allen G. Breed, File)
 The medical condition for a
troublingly low sexual appetite, called hypoactive sexual desire
disorder, has been recognized since the 1990s and is thought to
affect a significant portion of American women, according to
surveys. After the blockbuster success of Viagra for men in the
1990s, drugmakers began pouring money into research and potential
therapies for sexual dysfunction in women.
But diagnosing the condition is complicated because
of how many factors can affect libido, especially after menopause,
when falling hormone levels trigger a number of biological changes
and medical symptoms. Doctors are supposed to rule out a number of
other issues, including relationship problems, medical conditions,
depression and other mental disorders, before prescribing
medication.
The diagnosis is not universally accepted, and some psychologists
argue that low sex drive should not be considered a medical problem.
The FDA rejected Addyi twice prior to its 2015 approval, citing the
drug's modest effectiveness and worrisome side effects. The approval
came after a lobbying campaign by the company and its supporters,
Even the Score, which framed the lack of options for female libido
as a women's rights issue.
All contents © copyright 2025 Associated Press. All rights reserved |