US awards no-bid contract to Denmark scientists studying hepatitis B
vaccine in African babies
[December 20, 2025]
By MIKE STOBBE
NEW YORK (AP) — The Trump administration has awarded a $1.6 million,
no-bid contract to a Danish university to study hepatitis B vaccinations
on newborns in Africa that is raising ethical concerns.
The unusual contract was awarded to scientists who have been cited by
anti-vaccine activists and whose work has been questioned by leading
public health experts. Some experts have suggested the research plan is
unethical, because it will withhold vaccines that work from newborns at
significant risk of infection.
The contract did not undergo a customary ethics review, The Associated
Press has learned.
The Centers for Disease Control and Prevention awarded the grant to a
research team at the University of Southern Denmark that has been lauded
by U.S. Health Secretary Robert F. Kennedy Jr., according to a federal
notice posted this week.
One of the team's leaders is Christine Stabell Benn, a consultant for a
Kennedy-appointed committee that recently voted to stop recommending a
dose of hepatitis B vaccine for all U.S. newborns.
The study is to begin early next year in Guinea-Bissau, an impoverished
West African nation where hepatitis B infection is common. The
researchers are funded for five years to study 14,000 newborns.
It’s to be a randomized controlled trial, with some infants given the
hepatitis B vaccine at birth and some not. Children will be tracked for
death, illness and long-term developmental outcomes.

Most of the children will be followed for less than two years to look
for side effects, but the first 500 enrolled will be followed for five
years to look for behavior and brain development problems. There is no
placebo involved, according to a copy of the study protocol prepared
earlier this year that was obtained by the AP.
Hepatitis B can be passed from an infected mother to a baby. It also can
be spread by other infected people a baby comes in contact with.
Research and widespread medical consensus holds that the hepatitis B
vaccine protects newborns, so withholding it from some babies — in this
case, Black babies — has raised ethical alarms.
Medical evidence is clear that the vaccine protects infants from
developing liver disease and an early death. The well-documented
infection risk far outweighs hypothetical concerns about side effects,
said Dr. Boghuma K. Titanji, an Emory University infectious diseases
doctor.
She called the study “unconscionable,” and said it likely will
exacerbate existing vaccine hesitancy in Africa and elsewhere.

“There’s so much potential for this to be a harmful study,” said Titanji,
who is from Cameroon.
Benn did not respond to an email seeking comment about the proposal. An
automatic response said she is out of the office until early January.
But, in a statement, the research team said the study “will be the first
and likely the only one of its kind.”
They said it takes advantage of an unusual window of opportunity:
Guinea-Bissau doesn't currently recommended a birth dose of the hep B
vaccine, but the nation will be implementing universal vaccination of
newborns in 2027.
[to top of second column]
|
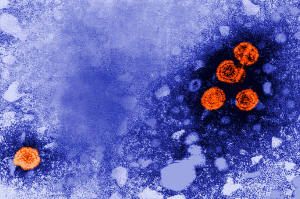
This 1981 electron microscope image made available by the U.S.
Centers for Disease Control and Prevention shows hepatitis B virus
particles, indicated in orange. (Dr. Erskine Palmer/CDC via AP,
File)
 Vaccine skeptics and opponents have
suggested that all the vaccine's possible side effects were
inadequately studied before the CDC began recommending it for
newborns in 1991. Public health experts counter that over more than
three decades no serious side effect has been documented.
The award is highly unusual. The CDC did not
announce a research funding opportunity and invite proposals.
The proposal was unsolicited and the award did not go through
customary review, said a CDC official with knowledge of the
decision. Department of Health and Human Services officials told CDC
officials to approve it and said HHS would provide special funding
for it, the CDC official said.
In private communications channels, CDC staffers were expressing
outrage about the award, said the official, who is not authorized to
talk about it and spoke on condition of anonymity.
Some of those CDC scientists have compared the work to the infamous
Tuskegee Study, which the agency oversaw in its later stages. In
that decades-long study, health workers withheld treatment from
unsuspecting Black men infected with syphilis so doctors could track
the horrible ravages of the disease.
Like Tuskegee, this study involves the prospect of researchers
watching people grow ill when a medical intervention could have kept
them healthy, Titanji echoed.
“It is an apt comparison,” she said.
The new study's researchers say the trial was approved by a national
ethics committee in Guinea-Bissau. But it did not undergo a
customary ethics review within the CDC, the agency official told the
AP.
In a statement, HHS spokesman Andrew Nixon said "we will ensure the
highest scientific and ethical standards are met.”
Public health scientists noted questions have been raised in the
past about research led by Benn and her husband, Peter Aaby, in
their Bandim Health Project.
Other Danish researchers who reviewed Aaby and Benn's work have
described questionable research practices. Earlier this year, former
CDC Director Dr. Tom Frieden wrote an editorial calling a 2017 study
co-authored by Aaby and Benn “fundamentally flawed."
Several researchers had harsh words about the latest award.
“Aaby and Benn are doing the Guinea-Bissau HBV vaccine depravation
trial," Carl Bergstrom, a University of Washington evolutionary
biologist, wrote in a post on Bluesky. "Did RFK Jr. just call up the
first name in the antivax yellow pages?”
Dr. Angela Rasmussen, a virus expert at the University of
Saskatchewan, said Kennedy was giving taxpayers' money to his
“cronies” for a “grossly unethical study that will expose African
babies to hep B for no reason.”
All contents © copyright 2025 Associated Press. All rights reserved

|