FDA OKs first blood test that can help diagnose Alzheimer's disease
[May 17, 2025]
By MATTHEW PERRONE
WASHINGTON (AP) — U.S. health officials on Friday endorsed the first
blood test that can help diagnose Alzheimer’s and identify patients who
may benefit from drugs that can modestly slow the memory-destroying
disease.
The test can aid doctors in determining whether a patient’s memory
problems are due to Alzheimer’s or a number of other medical conditions
that can cause cognitive difficulties. The Food and Drug Administration
cleared it for patients 55 and older who are showing early signs of the
disease.
More than 6 million people in the United States and millions more around
the world have Alzheimer’s, the most common form of dementia.
The new test, from Fujirebio Diagnostics, Inc., identifies a sticky
brain plaque, known as beta-amyloid, that is a key marker for
Alzheimer’s. Previously, the only FDA-approved methods for detecting
amyloid were invasive tests of spinal fluid or expensive PET scans.
The lower costs and convenience of a blood test could also help expand
use of two new drugs, Leqembi and Kisunla, which have been shown to
slightly slow the progression of Alzheimer’s by clearing amyloid from
the brain. Doctors are required to test patients for the plaque before
prescribing the drugs, which require regular IV infusions.
“Today’s clearance is an important step for Alzheimer’s disease
diagnosis, making it easier and potentially more accessible for U.S.
patients earlier in the disease,” said Dr. Michelle Tarver, of FDA’s
center for devices.

[to top of second column]
|
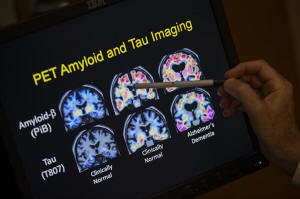
R. Scott Turner, Professor of Neurology and Director of the Memory
Disorder Center at Georgetown University Hospital, points to PET
scan results that are part of a study on Alzheimer's disease at
Georgetown University Hospital in Washington, May 19, 2015. (AP
Photo/Evan Vucci, File)
 A number of specialty hospitals and
laboratories have already developed their own in-house tests for
amyloid in recent years. But those tests aren’t reviewed by the FDA
and generally aren’t covered by insurance. Doctors have also had
little data to judge which tests are reliable and accurate, leading
to an unregulated marketplace that some have called a “wild west.”
Several larger diagnostic and drug companies are also developing
their own tests for FDA approval, including Roche, Eli Lilly and C2N
Diagnostics.
The tests can only be ordered by a doctor and aren’t intended for
people who don’t yet have any symptoms.
___
AP Medical Writer Lauran Neergaard contributed to this story
All contents © copyright 2025 Associated Press. All rights reserved |