Lifelong drugs for autoimmune diseases don't work well. Now scientists
are trying something new
[November 14, 2025]
By LAURAN NEERGAARD
Scientists are trying a revolutionary new approach to treat rheumatoid
arthritis, multiple sclerosis, lupus and other devastating autoimmune
diseases — by reprogramming patients’ out-of-whack immune systems.
When your body’s immune cells attack you instead of protecting you,
today’s treatments tamp down the friendly fire but they don’t fix what’s
causing it. Patients face a lifetime of pricey pills, shots or infusions
with some serious side effects — and too often the drugs aren’t enough
to keep their disease in check.
“We’re entering a new era,” said Dr. Maximilian Konig, a rheumatologist
at Johns Hopkins University who’s studying some of the possible new
treatments. They offer “the chance to control disease in a way we’ve
never seen before.”
How? Researchers are altering dysfunctional immune systems, not just
suppressing them, in a variety of ways that aim to be more potent and
more precise than current therapies.
They’re highly experimental and, because of potential side effects, so
far largely restricted to patients who’ve exhausted today’s treatments.
But people entering early-stage studies are grasping for hope.
“What the heck is wrong with my body?” Mileydy Gonzalez, 35, of New York
remembers crying, frustrated that nothing was helping her daily lupus
pain.
Diagnosed at 24, her disease was worsening, attacking her lungs and
kidneys. Gonzalez had trouble breathing, needed help to stand and walk
and couldn’t pick up her 3-year-old son when last July, her doctor at
NYU Langone Health suggested the hospital’s study using a treatment
adapted from cancer.
Gonzalez had never heard of that CAR-T therapy but decided, “I’m going
to trust you.” Over several months, she slowly regained energy and
strength.
“I can actually run, I can chase my kid,” said Gonzalez, who now is
pain- and pill-free. “I had forgotten what it was to be me.”

‘Living drugs’ reset rogue immune systems
CAR-T was developed to wipe out hard-to-treat blood cancers. But the
cells that go bad in leukemias and lymphomas — immune cells called B
cells — go awry in a different way in many autoimmune diseases.
Some U.S. studies in mice suggested CAR-T therapy might help those
diseases. Then in Germany, Dr. Georg Schett at the University of
Erlangen-Nuremberg tried it with a severely ill young woman who had
failed other lupus treatment. After one infusion, she’s been in
remission — with no other medicine — since March 2021.
Last month, Schett told a meeting of the American College of
Rheumatology how his team gradually treated a few dozen more patients,
with additional diseases such as myositis and scleroderma — and few
relapses so far.
Those early results were “shocking,” Hopkins’ Konig recalled.
They led to an explosion of clinical trials testing CAR-T therapy in the
U.S. and abroad for a growing list of autoimmune diseases.
How it works: Immune soldiers called T cells are filtered out of a
patient’s blood and sent to a lab, where they’re programmed to destroy
their B cell relatives. After some chemotherapy to wipe out additional
immune cells, millions of copies of those “living drugs” are infused
back into the patient.
While autoimmune drugs can target certain B cells, experts say they
can’t get rid of those hidden deep in the body. CAR-T therapy targets
both the problem B cells and healthy ones that might eventually run
amok. Schett theorizes that the deep depletion reboots the immune system
so when new B cells eventually form, they’re healthy.
Other ways to reprogram rogue cells
CAR-T is grueling, time consuming and costly, in part because it is
customized. A CAR-T cancer treatment can cost $500,000. Now some
companies are testing off-the-shelf versions, made in advance using
cells from healthy donors.
Another approach uses “peacekeeper” cells at the center of this year’s
Nobel Prize. Regulatory T cells are a rare subset of T cells that tamp
down inflammation and help hold back other cells that mistakenly attack
healthy tissue. Some biotech companies are engineering cells from
patients with rheumatoid arthritis and other diseases not to attack,
like CAR-T does, but to calm autoimmune reactions.
Scientists also are repurposing another cancer treatment, drugs called T
cell engagers, that don’t require custom engineering. These lab-made
antibodies act like a matchmaker. They redirect the body's existing T
cells to target antibody-producing B cells, said Erlangen's Dr. Ricardo
Grieshaber-Bouyer, who works with Schett and also studies possible
alternatives to CAR-T.
[to top of second column]
|
Research fellow Sachin Surwase shows an image of a pancreatic lymph
node from a mouse in the lab where he studies autoimmune diseases at
Johns Hopkins University in Baltimore, Md., Tuesday, May 13, 2025.
(AP Photo/David Goldman)
 Last month, Grieshaber-Bouyer
reported giving a course of one such drug, teclistamab, to 10
patients with a variety of diseases including Sjögren’s, myositis
and systemic sclerosis. All but one improved significantly and six
went into drug-free remission.
Next-generation precision options
Rather than wiping out swaths of the immune system, Hopkins’ Konig
aims to get more precise, targeting “only that very small population
of rogue cells that really causes the damage.”
B cells have identifiers, like biological barcodes, showing they can
produce faulty antibodies, Konig said. Researchers in his lab are
trying to engineer T cell engagers that would only mark “bad” B
cells for destruction, leaving healthy ones in place to fight
infection.
Nearby in another Hopkins lab, biomedical engineer Jordan Green is
crafting a way for the immune system to reprogram itself with the
help of instructions delivered by messenger RNA, or mRNA, the
genetic code used in COVID-19 vaccines.
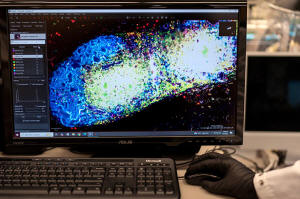
In Green's lab, a computer screen shines with brightly colored dots
that resemble a galaxy. It’s a biological map that shows
insulin-producing cells in the pancreas of a mouse. Red marks rogue
T cells that destroy insulin production. Yellow indicates those
peacemaker regulatory T cells — and they're outnumbered.
Green's team aims to use that mRNA to instruct certain immune
“generals” to curb the bad T cells and send in more peacemakers.
They package the mRNA in biodegradable nanoparticles that can be
injected like a drug. When the right immune cells get the messages,
the hope is they'd “divide, divide, divide and make a whole army of
healthy cells that then help treat the disease," Green said.
The researchers will know it's working if that galaxy-like map shows
less red and more yellow. Studies in people are still a few years
away.
Could you predict autoimmune diseases - and delay or prevent
them?
A drug for Type 1 diabetes “is forging the path,” said Dr. Kevin
Deane at the University of Colorado Anshutz.
Type 1 diabetes develops gradually, and blood tests can spot people
who are brewing it. A course of the drug teplizumab is approved to
delay the first symptoms, modulating rogue T cells and prolonging
insulin production.
Deane studies rheumatoid arthritis and hopes to find a similar way
to block the joint-destroying disease.
About 30% of people with a certain self-reactive antibody in their
blood will eventually develop RA. A new study tracked some of those
people for seven years, mapping immune changes leading to the
disease long before joints become swollen or painful.

Those changes are potential drug targets, Deane said. While
researchers hunt possible compounds to test, he’s leading another
study called StopRA: National to find and learn from more at-risk
people.
On all these fronts, there’s a tremendous amount of research left to
do — and no guarantees. There are questions about CAR-T's safety and
how long its effects last, but it is furthest along in testing.
Allie Rubin, 60, of Boca Raton, Florida, spent three decades
battling lupus, including scary hospitalizations when it attacked
her spinal cord. But she qualified for CAR-T when she also developed
lymphoma — and while a serious side effect delayed her recovery,
next month will mark two years without a sign of either cancer or
lupus.
“I just remember I woke up one day and thought, ‘Oh my god, I don’t
feel sick anymore,’” she said.
That kind of result has researchers optimistic.
"We’ve never been closer to getting to — and we don’t like to say it
— a potential cure,” said Hopkins' Konig. “I think the next 10 years
will dramatically change our field forever.”
All contents © copyright 2025 Associated Press. All rights reserved
 |