Eswatini is the first African country to get twice-yearly HIV prevention
shot
[November 19, 2025]
By FARAI MUTSAKA
HARARE, Zimbabwe (AP) — Eswatini on Tuesday became the first African
country to receive lenacapavir, the first twice-yearly HIV prevention
injection hailed by global health officials as a game-changer in the
fight against a virus that has killed tens of millions of people across
the continent.
Developed by Gilead Sciences, lenacapavir has demonstrated near-total
protection in clinical studies. Its rollout, initially planned for 10
high-risk African countries, is part of the U.S. President’s Emergency
Plan for AIDS Relief, or PEPFAR, in partnership with the Global Fund. By
2027, the initiative aims to benefit at least 2 million people in those
countries.
Daniel O’Day, chair and CEO of Gilead Sciences, described the Eswatini
rollout as “extraordinary” because “it’s the first time in history that
a new HIV medicine is reaching a country in sub-Saharan Africa in the
same year as approval of the United States” and because Eswatini “is the
country with the highest incidence of HIV in the world.” The U.S.
approved the drug in June.
The United States, whose deep cuts to foreign aid this year under
President Donald Trump have severely impacted Africa’s health programs,
initially planned to distribute 250,000 doses this year to the 10
countries. Zambia also received its first shipment Tuesday, while Gilead
seeks regulatory authority in Botswana, Kenya, Malawi, Namibia, Rwanda,
Tanzania, Uganda and Zimbabwe.

That was increased to 325,000 due to “early demand signs,” Brad Smith,
senior advisor for the Bureau of Global Health Security and Diplomacy,
told journalists.
The U.S. government has noted that over 25 million people across Africa
are living with HIV.
In Eswatini, a tiny kingdom in southern Africa, about 6,000 high-risk
people are set to benefit from the drug's initial rollout, primarily to
prevent HIV transmission from mothers to newborns. Home to roughly 1.2
million people, Eswatini currently has over 200,000 people living with
HIV, with most receiving treatment funded by PEPFAR, Smith said.
[to top of second column]
|
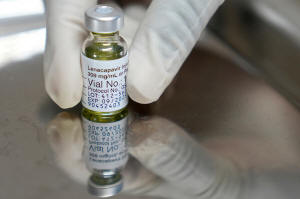
A pharmacist holds a vial of lenacapavir, an injectable HIV
prevention drug, at the Desmond Tutu Health Foundation's
Masiphumelele Research Site, in Cape Town, South Africa, July 23,
2024. (AP Photo/Nardus Engelbrecht, File)
 Eswatini, the world’s last absolute
monarchy with a documented record of human rights violations, is
also among the African countries participating in Trump’s
third-country deportation program, which has faced protests from
rights groups.
In July, the World Health Organization approved lenacapavir as an
additional HIV prevention option. UNAIDS has called long-acting
injectables a “fresh option” amid concerns that foreign funding cuts
could worsen infections.
South Africa’s health minister, Aaron Motsoaledi, recently called
lenacapavir “groundbreaking” but raised concerns over limited
supplies when South Africa begins its own rollout in April 2026.
Motsoaledi also welcomed Gilead’s steep price reduction from over
$28,000 per person annually in the U.S. to approximately $40 for
lower-income countries.
The rollout has fueled debate over access and manufacturing rights.
Civil society groups in South Africa, sub-Saharan Africa’s most
advanced economy, have criticized Gilead for excluding local
manufacturers from voluntary licensing agreements despite the
country’s role in clinical trials.
All contents © copyright 2025 Associated Press. All rights reserved
 |